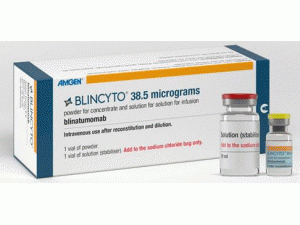
博纳吐单抗重组冻干粉注射剂Blincyto 35μg vial(Blinatumomab)
 药店国别:
产地国家:日本
处方药:是
所属类别: 35微克+附10毫升 1瓶/套件
包装规格: 35微克+附:10毫升 1瓶/套件
计价单位:套件
生产厂家中文参考译名:
生产厂家英文名:Asturias
原产地英文商品名:BLINCYTO(ビーリンサイト点滴静注用)35mcg+Appendix: 10mL 1bottles/KIT
原产地英文药品名:Blinatumomab(Genetical Recombination)
中文参考商品译名:BLINCYTO(ビーリンサイト点滴静注用)35微克+附:10毫升 1瓶/套件
中文参考药品译名:博纳吐单抗重组
曾用名:
简介:
部份中文博纳吐单抗重组处方资料(仅供参考)商品名:BLINCYTO英文名:Blinatumomab(Genetical Recombination)中文名:博纳吐单抗重组冻干粉注射剂生产商:安斯泰来制药药品简介Blinatumomab是一种全人源化双特异性抗体,结合到B细胞系表面表达的CD19和T细胞表面表达的CD3上。其通过用B细胞上的CD19与T细胞受体复合物的CD3连接,来激活内源性T细胞。Blinatumomab介导T细胞和肿瘤细胞之间的突触的形成,上调细胞粘附分子,产生细胞溶解蛋白,释放炎症性细胞因子,促进T细胞增殖,从而引起CD19+细胞定向溶解。ビーリンサイト点滴静注用35μg药效分类名抗恶性肿瘤剂/双重特异性抗体制剂批准日期:2014年12月商標名BLINCYTO一般名ブリナツモマブ(遺伝子組換え)Blinatumomab(Genetical Recombination)本質Blinatumomab是遗传因子组的一个锁链抗体(scFv-scFv),第1-111是鼠标抗人CD 19单克拉抗体的L链的可变区域,127-250是鼠标抗人CD 19单克朗抗体的H链的可变区域,256-374是鼠标抗人CD 3单锁抗体的H锁链的可变区域,392-498是由鼠标抗人CD 3单调抗体的L链的可变区域构成的。布里纳兹莫马夫是由504个氨基酸残基组成的蛋白质。分子量:约54 , 000批准条件1.在制定医药品风险管理计划的基础上,适当地使用。2.早期收集剂的安全性及有效性的数据,以合理使用本剂的适当措施。
药效药理
1.作用机制Blinatumomab是通过林克结合cd3和cd19的两种变体抗体的可变区域的基因重组蛋白。brittnimp是通过在t细胞细胞膜上发到的cd3和b细胞性肿瘤的细胞膜上连接到的cd19,通过架桥来激活t细胞,并被认为是对cd19阳性的肿瘤细胞进行伤害。
2.药理作用(1) in vitro测试在人末梢血单核体(pbmc)的存在下,印度人细胞性细胞性急性淋巴性白血病的nalm -6、kopn -8、sem细胞股等,显示出了增殖的作用。(2)in vivo试验布里纳兹蒙在与人pbmc一起在皮下移植的非肥胖型糖尿病/重度复合型免疫缺陷(nod/scid)鼠标中,表现出了抑制肿瘤增殖的作用。另外,britemomave表示,将nalm-6细胞的股份与pbmc一同在静脉内移植的nod/scid鼠标中延长了生存期限。
适应症
复发性的b细胞性急性淋巴性白血病
用法与用量
通常,作为转基因(基因转换),将以下的吸收量进行28天的持续点滴后,再进行14天的休药。把它作为一循环,最长5次循环。之后,作为转基因(基因转换),28天持续进行点滴治疗,之后再服用56天。将此作为一循环,最长4次循环。另外,根据患者的状态,适当地减肥。体重超过45公斤的情况:第一循环的第1~7天为1日9μg,此后每天为28μg。体重不足45公斤的情况:第1循环的第1~7天是一天5μg/m2(体表面积),之后是1天15μg/m2(体表层面积)。但是,体重不要超过45公斤以上的患者。
包装:
点滴静注用35μg:1瓶(输液稳定性液10mL1瓶/附件)
制造销售
阿斯特拉斯
博纳吐单抗重组冻干粉注射剂英文版说明书
Brand name:BLINCYTO for Drip Infusion 35mcgActive ingredient:Blinatumomab(genetical recombination)Dosage form:Lyophilized injectionPrint on wrapping:Effects of this medicineThis medicine helps cytotoxic T cells connect to leukemia cells (malignant B cells), adds damage against targetedmalignant cells and suppresses growth of cancer cells.It is usually used to treat relapsed or refractory B-cell acute lymphoblastic leukemia.Before using this medicine, be sure to tell your doctor and pharmacist・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.・If you are pregnant or breastfeeding.・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinaleffects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)Dosing schedule (How to take this medicine)・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional))・In general, you will receive this medicine by continuous intravenous infusion for 28 days, followed by a 14-daytreatment-free interval. This is counted as one cycle, and the cycle is repeated up to a maximum of five times.After this, you will receive this medicine by continuous intravenous infusion for 28 days, followed by a 56-daytreatment-free interval. This is counted as one cycle, and the cycle is repeated up to a maximum of four times.・Your doctor will eva luate your response to the treatment with this medicine after this medicine is beingadministered for a certain period of time, then decide the number of treatment cycles. Ask your doctor about thedetails of your treatment cycles.Precautions while taking this medicine・Refrain from driving and operating machinery or engaging in other hazardous activities while this medicine is beingadministered. This medicine may cause seizure or disturbance of consciousness.・If you have a possibility of pregnancy, avoid pregnancy while you are taking this medicine and for a certain period oftime after the last dose of this medicine.Possible adverse reactions to this medicineThe most commonly reported adverse reactions include fever, cytokine release syndrome, abdominal pain, headache,and anemia. If any of these symptoms occur, consult with your doctor or pharmacist.The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicatedin brackets. If any of these symptoms occur, see your doctor immediately.・headache, insomnia, dizziness [neurological events]・fever, chills, tiredness [infections]・nausea, headache, chest pain [cytokine release syndrome]・depressed level of consciousness, decreased urination, shortness of breath [tumor lysis syndrome]・palpitations, fever, nosebleeds [myelosuppression]・severe abdominal pain, back pain, vomiting [pancreatitis]The above symptoms do not describe all the adverse reactions to this medicine. Consult with yourdoctor or pharmacist if you notice any symptoms of concern other than those listed above.InjectionPublished: 11/2018The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approvaldetails may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It isimportant to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response,patients should understand their medication and cooperate with the treatment.
药店国别:
产地国家:日本
处方药:是
所属类别: 35微克+附10毫升 1瓶/套件
包装规格: 35微克+附:10毫升 1瓶/套件
计价单位:套件
生产厂家中文参考译名:
生产厂家英文名:Asturias
原产地英文商品名:BLINCYTO(ビーリンサイト点滴静注用)35mcg+Appendix: 10mL 1bottles/KIT
原产地英文药品名:Blinatumomab(Genetical Recombination)
中文参考商品译名:BLINCYTO(ビーリンサイト点滴静注用)35微克+附:10毫升 1瓶/套件
中文参考药品译名:博纳吐单抗重组
曾用名:
简介:
部份中文博纳吐单抗重组处方资料(仅供参考)商品名:BLINCYTO英文名:Blinatumomab(Genetical Recombination)中文名:博纳吐单抗重组冻干粉注射剂生产商:安斯泰来制药药品简介Blinatumomab是一种全人源化双特异性抗体,结合到B细胞系表面表达的CD19和T细胞表面表达的CD3上。其通过用B细胞上的CD19与T细胞受体复合物的CD3连接,来激活内源性T细胞。Blinatumomab介导T细胞和肿瘤细胞之间的突触的形成,上调细胞粘附分子,产生细胞溶解蛋白,释放炎症性细胞因子,促进T细胞增殖,从而引起CD19+细胞定向溶解。ビーリンサイト点滴静注用35μg药效分类名抗恶性肿瘤剂/双重特异性抗体制剂批准日期:2014年12月商標名BLINCYTO一般名ブリナツモマブ(遺伝子組換え)Blinatumomab(Genetical Recombination)本質Blinatumomab是遗传因子组的一个锁链抗体(scFv-scFv),第1-111是鼠标抗人CD 19单克拉抗体的L链的可变区域,127-250是鼠标抗人CD 19单克朗抗体的H链的可变区域,256-374是鼠标抗人CD 3单锁抗体的H锁链的可变区域,392-498是由鼠标抗人CD 3单调抗体的L链的可变区域构成的。布里纳兹莫马夫是由504个氨基酸残基组成的蛋白质。分子量:约54 , 000批准条件1.在制定医药品风险管理计划的基础上,适当地使用。2.早期收集剂的安全性及有效性的数据,以合理使用本剂的适当措施。
药效药理
1.作用机制Blinatumomab是通过林克结合cd3和cd19的两种变体抗体的可变区域的基因重组蛋白。brittnimp是通过在t细胞细胞膜上发到的cd3和b细胞性肿瘤的细胞膜上连接到的cd19,通过架桥来激活t细胞,并被认为是对cd19阳性的肿瘤细胞进行伤害。
2.药理作用(1) in vitro测试在人末梢血单核体(pbmc)的存在下,印度人细胞性细胞性急性淋巴性白血病的nalm -6、kopn -8、sem细胞股等,显示出了增殖的作用。(2)in vivo试验布里纳兹蒙在与人pbmc一起在皮下移植的非肥胖型糖尿病/重度复合型免疫缺陷(nod/scid)鼠标中,表现出了抑制肿瘤增殖的作用。另外,britemomave表示,将nalm-6细胞的股份与pbmc一同在静脉内移植的nod/scid鼠标中延长了生存期限。
适应症
复发性的b细胞性急性淋巴性白血病
用法与用量
通常,作为转基因(基因转换),将以下的吸收量进行28天的持续点滴后,再进行14天的休药。把它作为一循环,最长5次循环。之后,作为转基因(基因转换),28天持续进行点滴治疗,之后再服用56天。将此作为一循环,最长4次循环。另外,根据患者的状态,适当地减肥。体重超过45公斤的情况:第一循环的第1~7天为1日9μg,此后每天为28μg。体重不足45公斤的情况:第1循环的第1~7天是一天5μg/m2(体表面积),之后是1天15μg/m2(体表层面积)。但是,体重不要超过45公斤以上的患者。
包装:
点滴静注用35μg:1瓶(输液稳定性液10mL1瓶/附件)
制造销售
阿斯特拉斯
博纳吐单抗重组冻干粉注射剂英文版说明书
Brand name:BLINCYTO for Drip Infusion 35mcgActive ingredient:Blinatumomab(genetical recombination)Dosage form:Lyophilized injectionPrint on wrapping:Effects of this medicineThis medicine helps cytotoxic T cells connect to leukemia cells (malignant B cells), adds damage against targetedmalignant cells and suppresses growth of cancer cells.It is usually used to treat relapsed or refractory B-cell acute lymphoblastic leukemia.Before using this medicine, be sure to tell your doctor and pharmacist・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.・If you are pregnant or breastfeeding.・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinaleffects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)Dosing schedule (How to take this medicine)・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional))・In general, you will receive this medicine by continuous intravenous infusion for 28 days, followed by a 14-daytreatment-free interval. This is counted as one cycle, and the cycle is repeated up to a maximum of five times.After this, you will receive this medicine by continuous intravenous infusion for 28 days, followed by a 56-daytreatment-free interval. This is counted as one cycle, and the cycle is repeated up to a maximum of four times.・Your doctor will eva luate your response to the treatment with this medicine after this medicine is beingadministered for a certain period of time, then decide the number of treatment cycles. Ask your doctor about thedetails of your treatment cycles.Precautions while taking this medicine・Refrain from driving and operating machinery or engaging in other hazardous activities while this medicine is beingadministered. This medicine may cause seizure or disturbance of consciousness.・If you have a possibility of pregnancy, avoid pregnancy while you are taking this medicine and for a certain period oftime after the last dose of this medicine.Possible adverse reactions to this medicineThe most commonly reported adverse reactions include fever, cytokine release syndrome, abdominal pain, headache,and anemia. If any of these symptoms occur, consult with your doctor or pharmacist.The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicatedin brackets. If any of these symptoms occur, see your doctor immediately.・headache, insomnia, dizziness [neurological events]・fever, chills, tiredness [infections]・nausea, headache, chest pain [cytokine release syndrome]・depressed level of consciousness, decreased urination, shortness of breath [tumor lysis syndrome]・palpitations, fever, nosebleeds [myelosuppression]・severe abdominal pain, back pain, vomiting [pancreatitis]The above symptoms do not describe all the adverse reactions to this medicine. Consult with yourdoctor or pharmacist if you notice any symptoms of concern other than those listed above.InjectionPublished: 11/2018The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approvaldetails may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It isimportant to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response,patients should understand their medication and cooperate with the treatment.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博纳吐单抗重组冻干粉注射剂Blincyto 35μg vial(Blinatumomab)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博纳吐单抗重组冻干粉注射剂Blincyto 35μg vial(Blinatumomab)