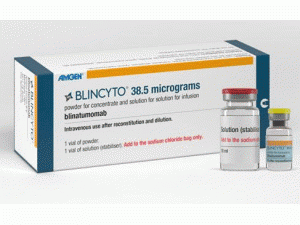
博纳吐单抗冻干粉注射剂Blincyto Injection 38.5mcg(Blinatumomab)
 药店国别:
产地国家:英国
处方药:是
所属类别: 38.5微克/瓶
包装规格: 38.5微克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Amgen
原产地英文商品名:BLINCYTO powder injection 38.5mcg/vial
原产地英文药品名:Blinatumomab
中文参考商品译名:BLINCYTO注射剂 38.5微克/瓶
中文参考药品译名:博纳吐单抗
曾用名:
简介:
2015年11月26日,生物技术巨头安进(Amgen)的BiTE免疫疗法Blincyto(blinatumomab)获欧盟有条件批准,用于费城染色体阴性(Ph-)复发性或难治性前体B细胞急性淋巴细胞白血病(ALL)成人患者的治疗。此次批准,使Blincyto成为欧洲批准的首个双特异性T细胞衔接器(BiTE)抗体药物。Blincyto的获批,是基于2项关键性II期研究(Study 211,Study 206)的积极数据,前一项研究中,Blincyto单药治疗组有高达42.9%的患者达到了完全缓解(CR)或部分血液学恢复的完全缓解(CRh)。Blincyto(blinatumomab)是全球首个BiTE免疫疗法,基于安进最先进的双特异性T细胞衔接系统(BiTE)开发,这是一种双特异性抗体,能够通过将肿瘤细胞上的CD19蛋白呈递给T细胞特异表达的CD3蛋白,进而激活免疫系统识别并杀灭肿瘤细胞。BiTE抗体技术代表了一种创新的免疫治疗方法,能够在很低浓度下起作用。目前,安进正在广泛的难治性肿瘤类型中。此前,FDA和EMA均已授予blinatumomab治疗多种类型血液癌症的孤儿药地位及突破性疗法认定,包括急性淋巴细胞白血病(ALL)、慢性淋巴细胞白血病(CLL)、毛细胞白血病(HCL)、幼淋巴细胞白血病(PLL)和惰性B细胞淋巴瘤、套细胞白血病(MCL)。包装规格:38.5ugx1Blincyto 38.5 micrograms
博纳吐单抗冻干粉注射剂英文版说明书
powder for concentrate and solution for solution for infusion.1. Name of the medicinal productBLINCYTO 38.5 micrograms powder for concentrate and solution for solution for infusion.2. Qualitative and quantitative compositionOne vial of powder contains 38.5 micrograms blinatumomab.Reconstitution with water for injections results in a final blinatumomab concentration of 12.5 micrograms/mL.Blinatumomab is produced in Chinese hamster ovary cells by recombinant DNA technology.For the full list of excipients, see section 6.1.3. Pharmaceutical formPowder for concentrate and solution for solution for infusion.BLINCYTO powder (powder for concentrate): White to off-white powder.Solution (stabiliser): Colourless-to-slightly yellow, clear solution with a pH of 7.0.4. Clinical particulars4.1 Therapeutic indicationsBLINCYTO is indicated for the treatment of adults with Philadelphia chromosome negative relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL).4.2 Posology and method of administrationTreatment should be initiated under the direction of and supervised by physicians experienced in the treatment of haematological malignancies.Hospitalisation is recommended for initiation at a minimum for the first 9 days of the first cycle and the first 2 days of the second cycle.In patients with a history or presence of clinically relevant central nervous system (CNS) pathology (see section 4.4), hospitalisation is recommended at a minimum for the first 14 days of the first cycle. In the second cycle, hospitalisation is recommended at a minimum for 2 days, and clinical judgment should be based on tolerance to BLINCYTO in the first cycle. Caution should be exercised as cases of late occurrence of first neurological events in the second cycle have been observed.For all subsequent cycle starts and reinitiation (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalisation is recommended.PosologyPatients may receive 2 cycles of treatment. A single cycle of treatment is 4 weeks of continuous infusion. Each cycle of treatment is separated by a 2 week treatment-free interval.Patients who have achieved complete remission (CR/CRh*) after 2 treatment cycles may receive up to 3 additional cycles of BLINCYTO consolidation treatment, based on an individual benefits-risks assessment.Recommended dose (for patients at least 45 kg in weight):Premedication and additional medication recommendationsDexamethasone 20 mg intravenous should be administered 1 hour prior to initiation of each cycle of BLINCYTO therapy.Anti-pyretic use (e.g. paracetamol) is recommended to reduce pyrexia during the first 48 hours of each treatment cycle.Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.Pre-phase treatment for patients with high tumour burdenFor patients with ≥ 50% leukaemic blasts or > 15,000/microlitre peripheral blood leukaemic blast counts treat with dexamethasone (not to exceed 24 mg/day).Dose adjustmentsConsideration to discontinue BLINCYTO temporarily or permanently as appropriate should be made in the case of the following severe (grade 3) or life-threatening (grade 4) toxicities (see section 4.4): cytokine release syndrome, tumour lysis syndrome, neurological toxicity, elevated liver enzymes and any other clinically relevant toxicities.If the interruption of treatment after an adverse event is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse event is longer than 7 days, start a new cycle. If the toxicity takes more than 14 days to resolve, discontinue BLINCYTO permanently, except if described differently in the table below.Special populationsElderlyNo dose adjustment is necessary in elderly patients (≥ 65 years of age), see section 5.1. There is limited experience with BLINCYTO in patients ≥ 75 years of age.Renal impairmentBased on pharmacokinetic analyses, dose adjustment is not necessary in patients with mild to moderate renal dysfunction (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe renal impairment.Hepatic impairmentBased on pharmacokinetic analyses, no effect of baseline liver function on blinatumomab exposure is expected and adjustment of the initial dose is not necessary (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe hepatic impairment.Paediatric populationThe safety and efficacy of BLINCYTO in paediatric patients have not yet been established.Currently available data are described in section 4.8 but no recommendation on a posology can be made.Method of administrationImportant note: Do not flush infusion lines into the patient, as it will cause an inadvertent bolus of BLINCYTO to be administered. BLINCYTO should be infused through a dedicated lumen.For instructions on the handling and preparation of the medicinal product before administration, see section 6.6.BLINCYTO solution for infusion is administered as a continuous intravenous infusion delivered at a constant flow rate using an infusion pump over a period of up to 96 hours.The BLINCYTO solution for infusion must be administered using intravenous tubing that contains an in-line, sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filter.A therapeutic dose of 9 mcg/day or 28 mcg/day should be administered to the patient by infusing a total of 240 mL BLINCYTO solution for infusion at one of 4 constant infusion rates and associated infusion durations:• Infusion rate of 10 mL/h for a duration of 24 hours• Infusion rate of 5 mL/h for a duration of 48 hours• Infusion rate of 3.3 mL/h for a duration of 72 hours• Infusion rate of 2.5 mL/h for a duration of 96 hoursThe choice of the infusion duration should be made by the treating physician considering the frequency of the infusion bag changes. The target therapeutic dose of BLINCYTO delivered does not change.Change of infusion bagThe infusion bag must be changed at least every 96 hours by a health care professional for sterility reasons.4.3 Contraindications- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.- Breast-feeding (see section 4.6).4.4 Special warnings and precautions for useNeurologic eventsNeurologic events including events with a fatal outcome have been observed. Grade 3 (CTCAE version 4.0) or higher (severe or life-threatening) neurologic events following initiation of blinatumomab administration included encephalopathy, seizures, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders.The median time from initiation of blinatumomab to onset of a neurologic event was 9 days. The majority of events resolved after treatment interruption.Elderly patients experienced a higher rate of neurological toxicities, including cognitive disorder, encephalopathy, and confusion.Patients with a medical history of neurologic signs and symptoms (such as dizziness, hypoaesthesia, hyporeflexia, tremor, dysaesthesia, paraesthesia, memory impairment) demonstrated a higher rate of neurologic events (such as tremor, dizziness, confusional state, encephalopathy and ataxia). The median time to onset of a neurologic event in these patients was 12 days.There is limited experience in patients with a history or presence of clinically relevant central nervous system (CNS) pathology (e.g. epilepsy, seizure, paresis, aphasia, stroke, severe brain injuries, dementia, Parkinson's disease, cerebellar disease, organic brain syndrome, psychosis) as they were excluded from clinical trials. There is a possibility of a higher risk of neurologic events in this population. The potential benefits of treatment should be carefully weighed against the risk of neurologic events and heightened caution should be exercised when administering BLINCYTO to these patients.There is limited experience with blinatumomab in patients with documented active ALL in the CNS or cerebrospinal fluid (CSF). However patients have been treated with blinatumomab in clinical studies after clearance of CSF blasts with CNS directed therapy (such as intrathecal chemotherapy). Therefore once the CSF is cleared, treatment with BLINCYTO may be initiated.It is recommended that a neurological examination be performed in patients prior to starting BLINCYTO therapy and that patients be clinically monitored for signs and symptoms of neurologic events (e.g. writing test). Management of these signs and symptoms to resolution may require either temporary interruption or permanent discontinuation of BLINCYTO (see section 4.2). In the event of a seizure, secondary prophylaxis with appropriate anticonvulsant medicinal products (e.g. levetiracetam) is recommended.InfectionsIn patients receiving blinatumomab, serious infections, including sepsis, pneumonia, bacteraemia, opportunistic infections and catheter site infections have been observed, some of which were life-threatening or fatal. Patients with Eastern Cooperative Oncology Group (ECOG) performance status at baseline of 2 experienced a higher incidence of serious infections compared to patients with ECOG performance status of < 2. There is limited experience with BLINCYTO in patients with an active uncontrolled infection.Patients receiving BLINCYTO should be clinically monitored for signs and symptoms of infection and treated appropriately. Management of infections may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Cytokine release syndrome and infusion reactionsCytokine release syndrome (CRS) which may be life-threatening or fatal (grade ≥ 4) has been reported in patients receiving BLINCYTO (see section 4.8).Serious adverse events that may be signs and symptoms of CRS included pyrexia, asthenia, headache, hypotension, total bilirubin increased, and nausea; uncommonly, these events led to BLINCYTO discontinuation. The median time to onset of a CRS event was 2 days. Patients should be closely monitored for signs or symptoms of these events.Disseminated intravascular coagulation (DIC) and capillary leak syndrome (CLS, e.g. hypotension, hypoalbuminaemia, oedema and haemoconcentration) have been commonly associated with CRS (see section 4.8). Patients experiencing capillary leak syndrome should be managed promptly.Haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) has been uncommonly reported in the setting of CRS.Infusion reactions may be clinically indistinguishable from manifestations of CRS (see section 4.8). The infusion reactions were generally rapid, occurring within 48 hours after initiating infusion. However some patients reported delayed onset of infusion reactions or in later cycles. Patients should be observed closely for infusion reactions, especially during the initiation of the first and second treatment cycles and treated appropriately. Anti-pyretic use (e.g. paracetamol) is recommended to help reduce pyrexia during the first 48 hours of each cycle. Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Tumour lysis syndromeTumour lysis syndrome (TLS), which may be life-threatening or fatal (grade ≥ 4) has been observed in patients receiving BLINCYTO.Appropriate prophylactic measures including aggressive hydration and anti-hyperuricaemic therapy (such as allopurinol or rasburicase) should be used for the prevention and treatment of TLS during BLINCYTO treatment, especially in patients with higher leukocytosis or a high tumour burden. Patients should be closely monitored for signs or symptoms of TLS, including renal function and fluid balance in the first 48 hours after the first infusion. In clinical studies, patients with moderate renal impairment showed an increased incidence of TLS compared with patients with mild renal impairment or normal renal function, Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Neutropenia and febrile neutropeniaNeutropenia and febrile neutropenia, including life threatening cases, have been observed in patients receiving BLINCYTO. Laboratory parameters (including, but not limited to white blood cell count and absolute neutrophil count) should be monitored routinely during BLINCYTO infusion, especially during the first 9 days of the first cycle, and treated appropriately.Elevated liver enzymesTreatment with BLINCYTO was associated with transient elevations in liver enzymes. The majority of the events were observed within the first week of treatment initiation and did not require interruption or discontinuation of BLINCYTO (see section 4.8).Monitoring of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during BLINCYTO treatment especially during the first 48 hours of the first 2 cycles should be performed. Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Leukoencephalopathy including progressive multifocal leukoencephalopathyCranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO, especially in patients with prior treatment with cranial irradiation and anti-leukaemic chemotherapy (including systemic high dose methotrexate or intrathecal cytarabine). The clinical significance of these imaging changes is unknown.Due to the potential for progressive multifocal leukoencephalopathy (PML), patients should be monitored for signs and symptoms. In case of suspicious events consider consultation with a neurologist, brain MRI and examination of cerebral spinal fluid (CSF), see section 4.8.ImmunisationsThe safety of immunisation with live viral vaccines during or following BLINCYTO therapy has not been studied. Vaccination with live virus vaccines is not recommended for at least 2 weeks prior to the start of BLINCYTO treatment, during treatment, and until recovery of B lymphocytes to normal ranges following last treatment cycle.Due to the potential depletion of B cells in newborns following exposure to blinatumomab during pregnancy, newborns should be monitored for B cell depletion and vaccinations with live virus vaccines should be postponed until the infant's B cell count has recovered (see section 4.6).ContraceptionWomen of childbearing potential have to use effective contraception during and for at least 48 hours, after treatment with BLINCYTO (see section 4.6).Medication errorsMedication errors have been observed with BLINCYTO treatment. It is very important that the instructions for preparation (including reconstitution and dilution) and administration are strictly followed to minimise medication errors (including underdose and overdose) (see section 4.2).Excipients with known effectThis medicinal product provides less than 1 mmol (23 mg) sodium over a 24 hour infusion i.e. “essentially sodium free”.4.5 Interaction with other medicinal products and other forms of interactionNo formal drug interaction studies have been performed. Results from an in vitro test in human hepatocytes suggest that blinatumomab did not affect CYP450 enzyme activities.Initiation of BLINCYTO treatment causes transient release of cytokines during the first days of treatment that may suppress CYP450 enzymes. Patients who are receiving medicinal products that are CYP450 and transporter substrates with a narrow therapeutic index should be monitored for adverse effects (e.g. warfarin) or drug concentrations (e.g. cyclosporine) during this time. The dose of the concomitant medicinal product should be adjusted as needed.4.6 Fertility, pregnancy and lactationPregnancyReproductive toxicity studies have not been conducted with blinatumomab. In an embryo-foetal developmental toxicity study conducted in mice, the murine surrogate molecule crossed the placenta and did not induce embryotoxicity, or teratogenicity (see section 5.3). The expected depletions of B and T cells were observed in the pregnant mice but haematological effects were not assessed in foetuses.There are no data from the use of blinatumomab in pregnant women.Blinatumomab should not be used during pregnancy unless the potential benefit outweighs the potential risk to the foetus.Women of childbearing potential have to use effective contraception during and for at least 48 hours after treatment with blinatumomab (see section 4.4).In case of exposure during pregnancy, depletion of B cells may be expected in newborns due to the pharmacological properties of the product. Consequently, newborns should be monitored for B cell depletion and vaccinations with live virus vaccines should be postponed until the infant's B cell count has recovered (see section 4.4).Breast-feedingIt is unknown whether blinatumomab or metabolites are excreted in human milk. Based on its pharmacological properties, a risk to the suckling child cannot be excluded. Consequently, as a precautionary measure, breast-feeding is contra-indicated during and for at least 48 hours after treatment with blinatumomab.FertilityNo studies have been conducted to eva luate the effects of blinatumomab on fertility. No adverse effects on male or female mouse reproductive organs in 13 week toxicity studies with the murine surrogate molecule (see section 5.3).4.7 Effects on ability to drive and use machinesBlinatumomab has major influence on the ability to drive and use machines. Confusion and disorientation, coordination and balance disorders, risk of seizures and disturbances in consciousness can occur (see section 4.4). Due to the potential for neurologic events, patients receiving blinatumomab should refrain from driving, engaging in hazardous occupations or activities such as driving or operating heavy or potentially dangerous machinery while blinatumomab is being administered. Patients must be advised that they may experience neurologic events.4.8 Undesirable effectsSummary of the safety profileThe adverse reactions described in this section were identified in the pivotal clinical study (N = 189).The most serious adverse reactions that may occur during blinatumomab treatment include: infections (31.7%), neurologic events (16.4%), neutropenia/febrile neutropenia (15.3%), cytokine release syndrome (0.5%), and tumour lysis syndrome (0.5%).The most common adverse reactions were: infusion-related reactions (67.2%), infections (63.0%), pyrexia (59.8%), headache (34.4%), febrile neutropenia (28%), peripheral oedema (25.9%), nausea (24.3%), hypokalaemia (23.8%), constipation (20.6%), anaemia (20.1%), cough (18.5%), diarrhoea (18.0%), tremor (17.5%), neutropenia (17.5%), abdominal pain (16.9%), insomnia (15.3%), fatigue (15.3%) and chills (15.3%).Tabulated list of adverse reactionsAdverse reactions are presented below by system organ class and frequency category. Frequency categories were determined from the crude incidence rate reported for each adverse reaction in the pivotal clinical study (N = 189). Within each system organ class, adverse reactions are presented in order of decreasing seriousness.a Additional information is provided in “Description of selected adverse reactions”b MedDRA high level group terms (MedDRA version 16.1)Description of selected adverse reactionsNeurologic eventsIn the pivotal clinical study (N = 189), 51.9% of patients experienced one or more neurologic adverse reactions (including psychiatric disorders), primarily involving the central nervous system. Serious and grade ≥ 3 neurologic adverse reactions were observed in 16.4% and 12.7% of patients respectively, of which the most common were encephalopathy, tremor, and confusional state. Fatal encephalopathy has been reported, however, the majority of neurologic events (74.5 %) were clinically reversible and resolved following interruption of BLINCYTO. The median time to onset of neurologic event was 9 days. For clinical management of neurologic events, see section 4.4.InfectionsLife-threatening or fatal (grade ≥ 4) viral, bacterial and fungal infections have been reported in patients treated with BLINCYTO. In addition, reactivations of virus infection (e.g. Polyoma (BK)) have been observed. Patients with ECOG performance status at baseline of 2 experienced a higher incidence of serious infections compared to patients with ECOG performance status of < 2. For clinical management of infections, see section 4.4.Cytokine release syndrome (CRS)In the pivotal clinical study (N = 189), serious CRS reactions were reported in 0.5% of patients with a median time to onset of 2 days. For clinical management of CRS, see section 4.4.Elevated liver enzymesIn the pivotal clinical study (N = 189), 27.5% of patients reported elevated liver enzymes. Serious and grade ≥ 3 adverse reactions (such as ALT increased, AST increased, and blood bilirubin increased) were observed in 2.1% and 15.3% of patients respectively. The median time to onset to the first event was 3 days from the start of BLINCYTO treatment initiation. The duration of hepatic adverse reactions has generally been brief and with rapid resolution, often when continuing uninterrupted treatment with BLINCYTO. For clinical management of elevated liver enzymes, see section 4.4.Leukoencephalopathy including progressive multifocal leukoencephalopathyLeukoencephalopathy has been reported. Patients with brain MRI/CT findings consistent with leukoencephalopathy experienced concurrent serious adverse events including confusional state, tremor, cognitive disorder, encephalopathy, and convulsion. Although there is a potential for the development of progressive multifocal leukoencephalopathy (PML), no case of PML has been reported in the pivotal study.Paediatric populationThere is limited experience in paediatric patients. BLINCYTO has been eva luated in paediatric patients with relapsed or refractory B-precursor ALL in a phase I/II dose escalation/eva luation study. At a dose higher than the recommended dose for adult patients, a case of fatal cardiac failure occurred in the setting of life-threatening cytokine release syndrome (CRS) and tumour lysis syndrome (TLS), see section 4.4.Other special populationsThere is limited experience with BLINCYTO in patients ≥ 75 years of age. Generally, safety was similar between elderly patients (≥ 65 years of age) and patients less than 65 years of age treated with BLINCYTO. However, elderly patients may be more susceptible to serious neurologic events such as cognitive disorder, encephalopathy and confusion.The safety of BLINCYTO has not been studied in patients with severe renal impairment.ImmunogenicityIn the pivotal clinical study (N = 189), less than 1.4% of patients treated with blinatumomab tested positive for binding and neutralising anti-blinatumomab antibodies. All patients who tested positive for binding antibodies also tested positive for neutralising anti-blinatumomab antibodies. Anti-blinatumomab antibody formation might affect pharmacokinetics of blinatumomab.If formation of anti-blinatumomab antibodies with a clinically significant effect is suspected, contact the Marketing Authorisation Holder to discuss antibody testing. Contact details are provided in section 6 of the package leaflet.Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:United KingdomYellow Card SchemeWebsite: www.mhra.gov.uk/yellowcardIrelandHPRA PharmacovigilanceEarlsfort TerraceIRL - Dublin 2Tel: +353 1 6764971Fax: +353 1 6762517Website: www.hpra.iee-mail: medsafety@hpra.ie4.9 OverdoseOverdoses have been observed including one patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration. Overdoses resulted in adverse reactions which were consistent with the reactions observed at the recommended therapeutic dose and included fever, tremors, and headache. In the event of overdose, the infusion should be temporarily interrupted and patients should be monitored. Reinitiation of BLINCYTO at the correct therapeutic dose should be considered when all toxicities have resolved and no earlier than 12 hours after interruption of the infusion (see section 4.2).5. Pharmacological properties5.1 Pharmacodynamic propertiesPharmacotherapeutic group: Antineoplastic agents, other Antineoplastic agents, ATC code: L01XC19.Mechanism of actionBlinatumomab is a bispecific T-cell engager antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T-cells. It activates endogenous T-cells by connecting CD3 in the T-cell receptor (TCR) complex with CD19 on benign and malignant B-cells. The anti-tumour activity of blinatumomab immunotherapy is not dependent on T-cells bearing a specific TCR or on peptide antigens presented by cancer cells, but is polyclonal in nature and independent of human leukocyte antigen (HLA) molecules on target cells. Blinatumomab mediates the formation of a cytolytic synapse between the T-cell and the tumour cell, releasing proteolytic enzymes to kill both proliferating and resting target cells. Blinatumomab is associated with transient upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T-cells, and results in elimination of CD19+ cells.Pharmacodynamic effectsConsistent immune-pharmacodynamic responses were observed in patients studied. During the continuous intravenous infusion over 4 weeks, the pharmacodynamic response was characterised by T-cell activation and initial redistribution, rapid peripheral B-cell depletion, and transient cytokine elevation.Peripheral T-cell redistribution (i.e., T-cell adhesion to blood vessel endothelium and/or transmigration into tissue) occurred after start of blinatumomab infusion or dose escalation. T-cell counts initially declined within 1 to 2 days and then returned to baseline levels within 7 to 14 days in the majority of patients. Increase of T-cell counts above baseline (T-cell expansion) was observed in few patients.Peripheral B-cell counts decreased rapidly to an undetectable level during treatment at doses ≥ 5 mcg/m2/day or ≥ 9 mcg/day in the majority of patients. No recovery of peripheral B-cell counts was observed during the 2-week treatment-free period between treatment cycles. Incomplete depletion of B-cells occurred at doses of 0.5 mcg/m2/day and 1.5 mcg/m2/day and in a few non-responders at higher doses.Cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ were measured and, IL-6, IL-10 and IFN-γ were most elevated. Transient elevation of cytokines was observed in the first two days following start of blinatumomab infusion. The elevated cytokine levels returned to baseline within 24 to 48 hours during the infusion. In subsequent treatment cycles, cytokine elevation occurred in fewer patients with lesser intensity compared to the initial 48 hours of the first treatment cycle.Clinical efficacy and safetyA total of 225 patients aged ≥ 18 years of age with relapsed or refractory B-precursor ALL were exposed to BLINCYTO during clinical trials.BLINCYTO was eva luated in an open-label, multicentre, single-arm phase II study of 189 patients. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-precursor ALL (relapsed with first remission duration of ≤ 12 months in first salvage, or relapsed or refractory after first salvage therapy, or relapsed within 12 months of allogeneic HSCT, and had ≥ 10% blasts in bone marrow).Patients were premedicated with a mandatory cerebrospinal fluid prophylaxis consisting of an intrathecal regimen according to institutional or national guidelines within 1 week prior to start of blinatumomab treatment. BLINCYTO was administered as a continuous intravenous infusion. In the first cycle, the initial dose was 9 mcg/day for week 1, then 28 mcg/day for the remaining 3 weeks. The target dose of 28 mcg/day was administered in cycle 2 and subsequent cycles starting on day 1 of each cycle. Dose adjustment was possible in the case of adverse events. The treated population included 189 patients who received at least 1 infusion of BLINCYTO; the mean number of cycles per patient was 1.6. Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO. Among treated patients, the median age was 39 years (range: 18 to 79 years, including 25 patients ≥ 65 years of age), 64 of 189 (33.9%) had undergone HSCT prior to receiving BLINCYTO and 32 of 189 (16.9%) had received more than 2 prior salvage therapies.The primary endpoint was the complete remission/complete remission with partial haematological recovery (CR/CRh*) rate within 2 cycles of treatment with BLINCYTO. Eighty-one of 189 (42.9%) patients achieved CR/CRh* within the first 2 treatment cycles with the majority of responses (64 of 81) occurring within 1 cycle of treatment. In the elderly population (≥ 65 years of age) 11 of 25 patients (44.0%) achieved CR/CRh* within the first 2 treatment cycles (see section 4.8 for safety in elderly). Four patients achieved CR during consolidation cycles, resulting in a cumulative CR rate of 35.4% (67/189; 95% CI: 28.6% - 42.7%). Thirty-two of 189 (17%) patients underwent allogeneic HSCT in CR/CRh* induced with BLINCYTO (see table 1).Table 1. Efficacy results in patients ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL)In a prespecified exploratory analysis, 60 of 73 MRD eva luable patients with CR/CRh* (82.2%) also had a MRD response (defined as MRD by PCR < 1 x 10-4).Patients with prior allogeneic HSCT had similar response rates to those without prior HSCT, older patients had similar response rates to younger patients, and no substantial difference was observed in remission rates based on the number of lines of prior salvage treatment.In patients with non-CNS/non-testes extramedullary disease (defined as at least 1 lesion ≥ 1.5 cm) at screening (N = 8/189) clinical response rates (25% [95% CI 3.2-65.1] were lower compared with patients with no evidence of extramedullary disease (N = 181, 43.6% [95%CI 36.3 - 51.2]) (see figure 1).Patients with the highest tumour burden as measured by the percentage of bone marrow blast cells at baseline (≥ 90%) still had a clinically meaningful response with a CR/CRh* rate of 21.6% (CI 12.9 – 32.7) (see figure 1). Patients with low tumour burden (< 50%) responded best to blinatumomab treatment with CR/CRh* rate of 72.9% (CI 59.7 – 83.6).Figure 1. Forest plot of CR/CRh* rate during the first two cycles for study MT103-211 (primary analysis set)In an open-label, multicentre, dose-escalation phase II study of 36 patients (≥ 18 years of age with B-precursor ALL relapsed after at least induction and consolidation or having refractory disease with > 5% blasts in bone marrow, ECOG performance status ≤ 2, life expectancy of ≥ 12 weeks, who did not have autologous hematopoietic stem cell transplantation (HSCT) within 6 weeks prior to start of blinatumomab treatment, allogeneic HSCT within 3 months prior to start of blinatumomab treatment, or previous treatment with blinatumomab), the safety and efficacy of blinatumomab were eva luated. Fifteen of 36 (41.7%) patients had undergone allogeneic HSCT prior to receiving BLINCYTO. The CR/CRh* rate was 69.4% (25 out of 36 patients: 15 [41.7%; 95% CI: 25.5% - 59.2%] CR; 10 [27.8%; 95% CI: 14.2% - 45.2%] CRh*). In the elderly population (≥ 65 years of age) 4 of 5 patients (80.0%) achieved CR/CRh* within 2 treatment cycles (see section 4.8 for safety in elderly). Twenty-two of 25 (88%) patients with haematologic complete remission also had minimal residual disease (MRD) responses (defined as MRD by PCR < 1 x 10-4). The median duration of remission was 8.9 months, and the median relapse-free survival (RFS) was 7.6 months. The median overall survival (OS) was 9.8 months.There is limited data in patients with late first relapse of B-precursor ALL defined as a relapse occurring more than 12 months after first remission or more than 12 months after HSCT in the first remission. In clinical studies, 88.9% (8/9) of patients with late first relapse as defined in the individual studies achieved CR/CRh* within the first 2 treatment cycles with 62.5% (6/9) achieving MRD response and 37.5 % (3/9) undergoing allogeneic HSCT after treatment with blinatumomab. The median overall survival (OS) was 17.7 months (CI 3.1 – not estimable).Paediatric populationThere is limited experience in paediatric patients, see section 4.8.The European Medicines Agency has deferred the obligation to submit the results of studies with BLINCYTO in children from 1 month to less than 18 years of age with acute lymphoblastic leukaemia (see section 4.2 for information on paediatric use).This medicinal product has been authorised under a so called 'conditional approval' scheme. This means that further evidence on this medicinal product is awaited. The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.5.2 Pharmacokinetic propertiesThe pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 90 mcg/m2/day (approximately equivalent to 9-162 mcg/day) in adult patients. Following continuous intravenous infusion, the steady state serum concentration (Css) was achieved within a day and remained stable over time. The increase in mean Css values was approximately proportional to the dose in the range tested. At the clinical doses of 9 mcg/day and 28 mcg/day for the treatment of relapsed/refractory ALL, the mean (SD) Css was 211 (258) pg/mL and 621 (502) pg/mL, respectively.DistributionThe estimated mean (SD) volume of distribution based on terminal phase (Vz) was 4.52 (2.89) L with the continuous intravenous infusion of blinatumomab.BiotransformationThe metabolic pathway of blinatumomab has not been characterised. Like other protein therapeutics, blinatumomab is expected to be degraded into small peptides and amino acids via catabolic pathways.EliminationThe estimated mean (SD) systemic clearance with continuous intravenous infusion in patients receiving blinatumomab in clinical studies was 2.92 (2.83) L/hour. The mean (SD) half-life was 2.11 (1.42) hours. Negligible amounts of blinatumomab were excreted in the urine at the tested clinical doses.Body weight, body surface area, gender and ageA population pharmacokinetic analysis was performed to eva luate the effects of demographic characteristics on blinatumomab pharmacokinetics. Results suggest that age (18 to 80 years), gender, body weight (44 to 134 kg), and body surface area (1.39 to 2.57) do not influence the pharmacokinetics of blinatumomab. There is very limited experience with blinatumomab in adults weighing less than 45 kg.Renal impairmentNo formal pharmacokinetic studies of blinatumomab have been conducted in patients with renal impairment.Pharmacokinetic analyses showed an approximately 2-fold difference in mean blinatumomab clearance values between patients with moderate renal dysfunction and normal renal function. However high inter-patient variability was discerned (CV% up to 95.6%), and clearance values in renal impaired patients were essentially within the range observed in patients with normal renal function, no clinically meaningful impact of renal function on clinical outcomes is expected.Hepatic impairmentNo formal pharmacokinetic studies of blinatumomab have been conducted in patients with hepatic impairment. Baseline ALT and AST levels were used to assess the effect of hepatic impairment on the clearance of blinatumomab. Population pharmacokinetic analysis suggested that there was no association between ALT or AST levels and the clearance of blinatumomab.Paediatric populationThere is limited experience in paediatric patients.5.3 Preclinical safety dataRepeat-dose toxicity studies conducted with blinatumomab and the murine surrogate revealed the expected pharmacologic effects (including release of cytokines, decreases in leukocyte counts, depletion of B-cells, decreases in T-cells, decreased cellularity in lymphoid tissues). These changes reversed after cessation of treatment.Reproductive toxicity studies have not been conducted with blinatumomab. In an embryo-foetal developmental toxicity study performed in mice, the murine surrogate crossed the placenta to a limited extent (foetal-to-maternal serum concentration ratio < 1%) and did not induce embryo-foetal toxicity or teratogenicity. The expected depletions of B- and T-cells were observed in the pregnant mice but haematological effects were not assessed in foetuses. No studies have been conducted to eva luate treatment-related effects on fertility. There were no effects on male or female reproductive organs in toxicity studies with the murine surrogate.6. Pharmaceutical particulars6.1 List of excipientsPowderCitric acid monohydrate (E330)Trehalose dihydrateLysine hydrochloridePolysorbate 80Sodium hydroxide (for pH-adjustment)Solution (stabiliser)Citric acid monohydrate (E330)Lysine hydrochloridePolysorbate 80Sodium hydroxide (for pH-adjustment)Water for injections6.2 IncompatibilitiesThis medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.6.3 Shelf lifeUnopened vials3 yearsReconstituted solutionChemical and physical in-use stability has been demonstrated for 24 hours at 2°C – 8°C or 4 hours at or below 27°C.From a microbiological point of view, unless the method of reconstituting precludes the risks of microbial contamination, the reconstituted solution should be diluted immediately. If not diluted immediately, in-use storage times and conditions are the responsibility of the user.Diluted solution (prepared infusion bag)Chemical and physical in-use stability has been demonstrated for 10 days at 2°C – 8°C or 96 hours at or below 27°C.From a microbiological point of view, the prepared infusion bags should be used immediately.If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C – 8°C, unless dilution has taken place in controlled and validated aseptic conditions.6.4 Special precautions for storageStore and transport refrigerated (2°C – 8°C).Do not freeze.Store the vials in the original package in order to protect from light.For storage conditions after reconstitution and dilution of the medicinal product, see section 6.3.6.5 Nature and contents of containerEach BLINCYTO pack contains 1 vial of powder for concentrate for solution for infusion and 1 vial of solution (stabiliser):• 38.5 micrograms blinatumomab powder in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap and• 10 mL solution in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap.6.6 Special precautions for disposal and other handlingAseptic preparationAseptic handling must be ensured when preparing the infusion. Preparation of BLINCYTO should be:- performed under aseptic conditions by trained personnel in accordance with good practice rules especially with respect to the aseptic preparation of parenteral products.- prepared in a laminar flow hood or biological safety cabinet using standard precautions for the safe handling of intravenous agents.It is very important that the instructions for preparation and administration provided in this section are strictly followed to minimise medication errors (including underdose and overdose).Special instructions to support accurate preparation• A solution (stabiliser) is provided inside the BLINCYTO package and is used to coat the pre-filled infusion bag prior to addition of reconstituted BLINCYTO. Do not use this solution (stabiliser)for reconstitution of BLINCYTO powder for concentrate.• The entire volume of the reconstituted and diluted BLINCYTO will be more than the volume to be administered to the patient (240 mL). This is to account for intravenous infusion line loss and to assure that the patient will receive the full dose of BLINCYTO.• When preparing an infusion bag, remove all air from infusion bag. This is particularly important when using an ambulatory infusion pump.• Use the specific volumes described in the reconstitution and dilution instructions below to minimise errors in calculation.Other instructions• BLINCYTO is compatible with polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.• Pump specifications: The infusion pump to administer BLINCYTO solution for infusion should be programmable, lockable and have an alarm. Elastomeric pumps should not be used.• Any unused medicinal product or waste material should be disposed of in accordance with local requirements.Preparation of the solution for infusionSpecific reconstitution and dilution instructions are provided for each dose and infusion time. Verify the prescribed dose and infusion time of BLINCYTO and identify the appropriate dosing preparation section listed below. Follow the steps for reconstituting BLINCYTO and preparing the infusion bag.a) for 9 mcg/day infused over 24 hours at a rate of 10 mL/hb) for 9 mcg/day infused over 48 hours at a rate of 5 mL/hc) for 9 mcg/day infused over 72 hours at a rate of 3.3 mL/hd) for 9 mcg/day infused over 96 hours at a rate of 2.5 mL/he) for 28 mcg/day infused over 24 hours at a rate of 10 mL/hf) for 28 mcg/day infused over 48 hours at a rate of 5 mL/hg) for 28 mcg/day infused over 72 hours at a rate of 3.3 mL/hh) for 28 mcg/day infused over 96 hours at a rate of 2.5 mL/hBefore preparation, ensure you have the following supplies ready:These supplies are also required, but not included in the package• Sterile single-use disposable syringes• 21-23 gauge needle(s) (recommended)• Water for injections• Infusion bag with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection;o To minimise the number of aseptic transfers, use a 250 mL pre-filled infusion bag. BLINCYTO dose calculations are based on a usual overfill volume of 265 to 275 mL sodium chloride 9 mg/mL (0.9%) solution for injection.o Use only polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.• Polyolefin, PVC non-DEHP, or EVA intravenous tubing with a sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filtero Ensure that the tubing is compatible with the infusion pump.a) Preparation of BLINCYTO 9 mcg/day infused over 24 hours at a rate of 10 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 0.83 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.b) Preparation of BLINCYTO 9 mcg/day infused over 48 hours at a rate of 5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 1.7 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.c) Preparation of BLINCYTO 9 mcg/day infused over 72 hours at a rate of 3.3 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 2.5 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.d) Preparation of BLINCYTO 9 mcg/day infused over 96 hours at a rate of 2.5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use two vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 3.5 mL of reconstituted BLINCYTO into the infusion bag (2.0 mL from one vial and the remaining 1.5 mL from the second vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.e) Preparation of BLINCYTO 28 mcg/day infused over 24 hours at a rate of 10 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 2.6 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.f) Preparation of BLINCYTO 28 mcg/day infused over 48 hours at a rate of 5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use two vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 5.2 mL of reconstituted BLINCYTO into the infusion bag (2.7 mL from one vial and the remaining 2.5 mL from the second vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.g) Preparation of BLINCYTO 28 mcg/day infused over 72 hours at a rate of 3.3 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use three vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 8 mL of reconstituted BLINCYTO into the infusion bag (2.8 mL from each of the first two vials and the remaining 2.4 mL from the third vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.h) Preparation of BLINCYTO 28 mcg/day infused over 96 hours at a rate of 2.5 mL/h1. Use an infusion bag pre-filled with250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use four vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 10.7 mL of reconstituted BLINCYTO into the infusion bag (2.8 mL from each of the first three vials and the remaining 2.3 mL from the fourth vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.For instructions on administration, see section 4.2.7. Marketing authorisation holderAmgen Europe B.V.Minervum 70614817 ZK BredaThe Netherlands8. Marketing authorisation number(s)EU/1/15/1047/0019. Date of first authorisation/renewal of the authorisationDate of first authorisation: 23 November 201510. Date of revision of the textNovember 2015Detailed information on this medicinal product is available on the website of the European Medicines Agency https://www.ema.europa.eu
药店国别:
产地国家:英国
处方药:是
所属类别: 38.5微克/瓶
包装规格: 38.5微克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Amgen
原产地英文商品名:BLINCYTO powder injection 38.5mcg/vial
原产地英文药品名:Blinatumomab
中文参考商品译名:BLINCYTO注射剂 38.5微克/瓶
中文参考药品译名:博纳吐单抗
曾用名:
简介:
2015年11月26日,生物技术巨头安进(Amgen)的BiTE免疫疗法Blincyto(blinatumomab)获欧盟有条件批准,用于费城染色体阴性(Ph-)复发性或难治性前体B细胞急性淋巴细胞白血病(ALL)成人患者的治疗。此次批准,使Blincyto成为欧洲批准的首个双特异性T细胞衔接器(BiTE)抗体药物。Blincyto的获批,是基于2项关键性II期研究(Study 211,Study 206)的积极数据,前一项研究中,Blincyto单药治疗组有高达42.9%的患者达到了完全缓解(CR)或部分血液学恢复的完全缓解(CRh)。Blincyto(blinatumomab)是全球首个BiTE免疫疗法,基于安进最先进的双特异性T细胞衔接系统(BiTE)开发,这是一种双特异性抗体,能够通过将肿瘤细胞上的CD19蛋白呈递给T细胞特异表达的CD3蛋白,进而激活免疫系统识别并杀灭肿瘤细胞。BiTE抗体技术代表了一种创新的免疫治疗方法,能够在很低浓度下起作用。目前,安进正在广泛的难治性肿瘤类型中。此前,FDA和EMA均已授予blinatumomab治疗多种类型血液癌症的孤儿药地位及突破性疗法认定,包括急性淋巴细胞白血病(ALL)、慢性淋巴细胞白血病(CLL)、毛细胞白血病(HCL)、幼淋巴细胞白血病(PLL)和惰性B细胞淋巴瘤、套细胞白血病(MCL)。包装规格:38.5ugx1Blincyto 38.5 micrograms
博纳吐单抗冻干粉注射剂英文版说明书
powder for concentrate and solution for solution for infusion.1. Name of the medicinal productBLINCYTO 38.5 micrograms powder for concentrate and solution for solution for infusion.2. Qualitative and quantitative compositionOne vial of powder contains 38.5 micrograms blinatumomab.Reconstitution with water for injections results in a final blinatumomab concentration of 12.5 micrograms/mL.Blinatumomab is produced in Chinese hamster ovary cells by recombinant DNA technology.For the full list of excipients, see section 6.1.3. Pharmaceutical formPowder for concentrate and solution for solution for infusion.BLINCYTO powder (powder for concentrate): White to off-white powder.Solution (stabiliser): Colourless-to-slightly yellow, clear solution with a pH of 7.0.4. Clinical particulars4.1 Therapeutic indicationsBLINCYTO is indicated for the treatment of adults with Philadelphia chromosome negative relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL).4.2 Posology and method of administrationTreatment should be initiated under the direction of and supervised by physicians experienced in the treatment of haematological malignancies.Hospitalisation is recommended for initiation at a minimum for the first 9 days of the first cycle and the first 2 days of the second cycle.In patients with a history or presence of clinically relevant central nervous system (CNS) pathology (see section 4.4), hospitalisation is recommended at a minimum for the first 14 days of the first cycle. In the second cycle, hospitalisation is recommended at a minimum for 2 days, and clinical judgment should be based on tolerance to BLINCYTO in the first cycle. Caution should be exercised as cases of late occurrence of first neurological events in the second cycle have been observed.For all subsequent cycle starts and reinitiation (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalisation is recommended.PosologyPatients may receive 2 cycles of treatment. A single cycle of treatment is 4 weeks of continuous infusion. Each cycle of treatment is separated by a 2 week treatment-free interval.Patients who have achieved complete remission (CR/CRh*) after 2 treatment cycles may receive up to 3 additional cycles of BLINCYTO consolidation treatment, based on an individual benefits-risks assessment.Recommended dose (for patients at least 45 kg in weight):Premedication and additional medication recommendationsDexamethasone 20 mg intravenous should be administered 1 hour prior to initiation of each cycle of BLINCYTO therapy.Anti-pyretic use (e.g. paracetamol) is recommended to reduce pyrexia during the first 48 hours of each treatment cycle.Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.Pre-phase treatment for patients with high tumour burdenFor patients with ≥ 50% leukaemic blasts or > 15,000/microlitre peripheral blood leukaemic blast counts treat with dexamethasone (not to exceed 24 mg/day).Dose adjustmentsConsideration to discontinue BLINCYTO temporarily or permanently as appropriate should be made in the case of the following severe (grade 3) or life-threatening (grade 4) toxicities (see section 4.4): cytokine release syndrome, tumour lysis syndrome, neurological toxicity, elevated liver enzymes and any other clinically relevant toxicities.If the interruption of treatment after an adverse event is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse event is longer than 7 days, start a new cycle. If the toxicity takes more than 14 days to resolve, discontinue BLINCYTO permanently, except if described differently in the table below.Special populationsElderlyNo dose adjustment is necessary in elderly patients (≥ 65 years of age), see section 5.1. There is limited experience with BLINCYTO in patients ≥ 75 years of age.Renal impairmentBased on pharmacokinetic analyses, dose adjustment is not necessary in patients with mild to moderate renal dysfunction (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe renal impairment.Hepatic impairmentBased on pharmacokinetic analyses, no effect of baseline liver function on blinatumomab exposure is expected and adjustment of the initial dose is not necessary (see section 5.2). The safety and efficacy of BLINCYTO have not been studied in patients with severe hepatic impairment.Paediatric populationThe safety and efficacy of BLINCYTO in paediatric patients have not yet been established.Currently available data are described in section 4.8 but no recommendation on a posology can be made.Method of administrationImportant note: Do not flush infusion lines into the patient, as it will cause an inadvertent bolus of BLINCYTO to be administered. BLINCYTO should be infused through a dedicated lumen.For instructions on the handling and preparation of the medicinal product before administration, see section 6.6.BLINCYTO solution for infusion is administered as a continuous intravenous infusion delivered at a constant flow rate using an infusion pump over a period of up to 96 hours.The BLINCYTO solution for infusion must be administered using intravenous tubing that contains an in-line, sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filter.A therapeutic dose of 9 mcg/day or 28 mcg/day should be administered to the patient by infusing a total of 240 mL BLINCYTO solution for infusion at one of 4 constant infusion rates and associated infusion durations:• Infusion rate of 10 mL/h for a duration of 24 hours• Infusion rate of 5 mL/h for a duration of 48 hours• Infusion rate of 3.3 mL/h for a duration of 72 hours• Infusion rate of 2.5 mL/h for a duration of 96 hoursThe choice of the infusion duration should be made by the treating physician considering the frequency of the infusion bag changes. The target therapeutic dose of BLINCYTO delivered does not change.Change of infusion bagThe infusion bag must be changed at least every 96 hours by a health care professional for sterility reasons.4.3 Contraindications- Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.- Breast-feeding (see section 4.6).4.4 Special warnings and precautions for useNeurologic eventsNeurologic events including events with a fatal outcome have been observed. Grade 3 (CTCAE version 4.0) or higher (severe or life-threatening) neurologic events following initiation of blinatumomab administration included encephalopathy, seizures, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders.The median time from initiation of blinatumomab to onset of a neurologic event was 9 days. The majority of events resolved after treatment interruption.Elderly patients experienced a higher rate of neurological toxicities, including cognitive disorder, encephalopathy, and confusion.Patients with a medical history of neurologic signs and symptoms (such as dizziness, hypoaesthesia, hyporeflexia, tremor, dysaesthesia, paraesthesia, memory impairment) demonstrated a higher rate of neurologic events (such as tremor, dizziness, confusional state, encephalopathy and ataxia). The median time to onset of a neurologic event in these patients was 12 days.There is limited experience in patients with a history or presence of clinically relevant central nervous system (CNS) pathology (e.g. epilepsy, seizure, paresis, aphasia, stroke, severe brain injuries, dementia, Parkinson's disease, cerebellar disease, organic brain syndrome, psychosis) as they were excluded from clinical trials. There is a possibility of a higher risk of neurologic events in this population. The potential benefits of treatment should be carefully weighed against the risk of neurologic events and heightened caution should be exercised when administering BLINCYTO to these patients.There is limited experience with blinatumomab in patients with documented active ALL in the CNS or cerebrospinal fluid (CSF). However patients have been treated with blinatumomab in clinical studies after clearance of CSF blasts with CNS directed therapy (such as intrathecal chemotherapy). Therefore once the CSF is cleared, treatment with BLINCYTO may be initiated.It is recommended that a neurological examination be performed in patients prior to starting BLINCYTO therapy and that patients be clinically monitored for signs and symptoms of neurologic events (e.g. writing test). Management of these signs and symptoms to resolution may require either temporary interruption or permanent discontinuation of BLINCYTO (see section 4.2). In the event of a seizure, secondary prophylaxis with appropriate anticonvulsant medicinal products (e.g. levetiracetam) is recommended.InfectionsIn patients receiving blinatumomab, serious infections, including sepsis, pneumonia, bacteraemia, opportunistic infections and catheter site infections have been observed, some of which were life-threatening or fatal. Patients with Eastern Cooperative Oncology Group (ECOG) performance status at baseline of 2 experienced a higher incidence of serious infections compared to patients with ECOG performance status of < 2. There is limited experience with BLINCYTO in patients with an active uncontrolled infection.Patients receiving BLINCYTO should be clinically monitored for signs and symptoms of infection and treated appropriately. Management of infections may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Cytokine release syndrome and infusion reactionsCytokine release syndrome (CRS) which may be life-threatening or fatal (grade ≥ 4) has been reported in patients receiving BLINCYTO (see section 4.8).Serious adverse events that may be signs and symptoms of CRS included pyrexia, asthenia, headache, hypotension, total bilirubin increased, and nausea; uncommonly, these events led to BLINCYTO discontinuation. The median time to onset of a CRS event was 2 days. Patients should be closely monitored for signs or symptoms of these events.Disseminated intravascular coagulation (DIC) and capillary leak syndrome (CLS, e.g. hypotension, hypoalbuminaemia, oedema and haemoconcentration) have been commonly associated with CRS (see section 4.8). Patients experiencing capillary leak syndrome should be managed promptly.Haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) has been uncommonly reported in the setting of CRS.Infusion reactions may be clinically indistinguishable from manifestations of CRS (see section 4.8). The infusion reactions were generally rapid, occurring within 48 hours after initiating infusion. However some patients reported delayed onset of infusion reactions or in later cycles. Patients should be observed closely for infusion reactions, especially during the initiation of the first and second treatment cycles and treated appropriately. Anti-pyretic use (e.g. paracetamol) is recommended to help reduce pyrexia during the first 48 hours of each cycle. Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Tumour lysis syndromeTumour lysis syndrome (TLS), which may be life-threatening or fatal (grade ≥ 4) has been observed in patients receiving BLINCYTO.Appropriate prophylactic measures including aggressive hydration and anti-hyperuricaemic therapy (such as allopurinol or rasburicase) should be used for the prevention and treatment of TLS during BLINCYTO treatment, especially in patients with higher leukocytosis or a high tumour burden. Patients should be closely monitored for signs or symptoms of TLS, including renal function and fluid balance in the first 48 hours after the first infusion. In clinical studies, patients with moderate renal impairment showed an increased incidence of TLS compared with patients with mild renal impairment or normal renal function, Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Neutropenia and febrile neutropeniaNeutropenia and febrile neutropenia, including life threatening cases, have been observed in patients receiving BLINCYTO. Laboratory parameters (including, but not limited to white blood cell count and absolute neutrophil count) should be monitored routinely during BLINCYTO infusion, especially during the first 9 days of the first cycle, and treated appropriately.Elevated liver enzymesTreatment with BLINCYTO was associated with transient elevations in liver enzymes. The majority of the events were observed within the first week of treatment initiation and did not require interruption or discontinuation of BLINCYTO (see section 4.8).Monitoring of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during BLINCYTO treatment especially during the first 48 hours of the first 2 cycles should be performed. Management of these events may require either temporary interruption or discontinuation of BLINCYTO (see section 4.2).Leukoencephalopathy including progressive multifocal leukoencephalopathyCranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO, especially in patients with prior treatment with cranial irradiation and anti-leukaemic chemotherapy (including systemic high dose methotrexate or intrathecal cytarabine). The clinical significance of these imaging changes is unknown.Due to the potential for progressive multifocal leukoencephalopathy (PML), patients should be monitored for signs and symptoms. In case of suspicious events consider consultation with a neurologist, brain MRI and examination of cerebral spinal fluid (CSF), see section 4.8.ImmunisationsThe safety of immunisation with live viral vaccines during or following BLINCYTO therapy has not been studied. Vaccination with live virus vaccines is not recommended for at least 2 weeks prior to the start of BLINCYTO treatment, during treatment, and until recovery of B lymphocytes to normal ranges following last treatment cycle.Due to the potential depletion of B cells in newborns following exposure to blinatumomab during pregnancy, newborns should be monitored for B cell depletion and vaccinations with live virus vaccines should be postponed until the infant's B cell count has recovered (see section 4.6).ContraceptionWomen of childbearing potential have to use effective contraception during and for at least 48 hours, after treatment with BLINCYTO (see section 4.6).Medication errorsMedication errors have been observed with BLINCYTO treatment. It is very important that the instructions for preparation (including reconstitution and dilution) and administration are strictly followed to minimise medication errors (including underdose and overdose) (see section 4.2).Excipients with known effectThis medicinal product provides less than 1 mmol (23 mg) sodium over a 24 hour infusion i.e. “essentially sodium free”.4.5 Interaction with other medicinal products and other forms of interactionNo formal drug interaction studies have been performed. Results from an in vitro test in human hepatocytes suggest that blinatumomab did not affect CYP450 enzyme activities.Initiation of BLINCYTO treatment causes transient release of cytokines during the first days of treatment that may suppress CYP450 enzymes. Patients who are receiving medicinal products that are CYP450 and transporter substrates with a narrow therapeutic index should be monitored for adverse effects (e.g. warfarin) or drug concentrations (e.g. cyclosporine) during this time. The dose of the concomitant medicinal product should be adjusted as needed.4.6 Fertility, pregnancy and lactationPregnancyReproductive toxicity studies have not been conducted with blinatumomab. In an embryo-foetal developmental toxicity study conducted in mice, the murine surrogate molecule crossed the placenta and did not induce embryotoxicity, or teratogenicity (see section 5.3). The expected depletions of B and T cells were observed in the pregnant mice but haematological effects were not assessed in foetuses.There are no data from the use of blinatumomab in pregnant women.Blinatumomab should not be used during pregnancy unless the potential benefit outweighs the potential risk to the foetus.Women of childbearing potential have to use effective contraception during and for at least 48 hours after treatment with blinatumomab (see section 4.4).In case of exposure during pregnancy, depletion of B cells may be expected in newborns due to the pharmacological properties of the product. Consequently, newborns should be monitored for B cell depletion and vaccinations with live virus vaccines should be postponed until the infant's B cell count has recovered (see section 4.4).Breast-feedingIt is unknown whether blinatumomab or metabolites are excreted in human milk. Based on its pharmacological properties, a risk to the suckling child cannot be excluded. Consequently, as a precautionary measure, breast-feeding is contra-indicated during and for at least 48 hours after treatment with blinatumomab.FertilityNo studies have been conducted to eva luate the effects of blinatumomab on fertility. No adverse effects on male or female mouse reproductive organs in 13 week toxicity studies with the murine surrogate molecule (see section 5.3).4.7 Effects on ability to drive and use machinesBlinatumomab has major influence on the ability to drive and use machines. Confusion and disorientation, coordination and balance disorders, risk of seizures and disturbances in consciousness can occur (see section 4.4). Due to the potential for neurologic events, patients receiving blinatumomab should refrain from driving, engaging in hazardous occupations or activities such as driving or operating heavy or potentially dangerous machinery while blinatumomab is being administered. Patients must be advised that they may experience neurologic events.4.8 Undesirable effectsSummary of the safety profileThe adverse reactions described in this section were identified in the pivotal clinical study (N = 189).The most serious adverse reactions that may occur during blinatumomab treatment include: infections (31.7%), neurologic events (16.4%), neutropenia/febrile neutropenia (15.3%), cytokine release syndrome (0.5%), and tumour lysis syndrome (0.5%).The most common adverse reactions were: infusion-related reactions (67.2%), infections (63.0%), pyrexia (59.8%), headache (34.4%), febrile neutropenia (28%), peripheral oedema (25.9%), nausea (24.3%), hypokalaemia (23.8%), constipation (20.6%), anaemia (20.1%), cough (18.5%), diarrhoea (18.0%), tremor (17.5%), neutropenia (17.5%), abdominal pain (16.9%), insomnia (15.3%), fatigue (15.3%) and chills (15.3%).Tabulated list of adverse reactionsAdverse reactions are presented below by system organ class and frequency category. Frequency categories were determined from the crude incidence rate reported for each adverse reaction in the pivotal clinical study (N = 189). Within each system organ class, adverse reactions are presented in order of decreasing seriousness.a Additional information is provided in “Description of selected adverse reactions”b MedDRA high level group terms (MedDRA version 16.1)Description of selected adverse reactionsNeurologic eventsIn the pivotal clinical study (N = 189), 51.9% of patients experienced one or more neurologic adverse reactions (including psychiatric disorders), primarily involving the central nervous system. Serious and grade ≥ 3 neurologic adverse reactions were observed in 16.4% and 12.7% of patients respectively, of which the most common were encephalopathy, tremor, and confusional state. Fatal encephalopathy has been reported, however, the majority of neurologic events (74.5 %) were clinically reversible and resolved following interruption of BLINCYTO. The median time to onset of neurologic event was 9 days. For clinical management of neurologic events, see section 4.4.InfectionsLife-threatening or fatal (grade ≥ 4) viral, bacterial and fungal infections have been reported in patients treated with BLINCYTO. In addition, reactivations of virus infection (e.g. Polyoma (BK)) have been observed. Patients with ECOG performance status at baseline of 2 experienced a higher incidence of serious infections compared to patients with ECOG performance status of < 2. For clinical management of infections, see section 4.4.Cytokine release syndrome (CRS)In the pivotal clinical study (N = 189), serious CRS reactions were reported in 0.5% of patients with a median time to onset of 2 days. For clinical management of CRS, see section 4.4.Elevated liver enzymesIn the pivotal clinical study (N = 189), 27.5% of patients reported elevated liver enzymes. Serious and grade ≥ 3 adverse reactions (such as ALT increased, AST increased, and blood bilirubin increased) were observed in 2.1% and 15.3% of patients respectively. The median time to onset to the first event was 3 days from the start of BLINCYTO treatment initiation. The duration of hepatic adverse reactions has generally been brief and with rapid resolution, often when continuing uninterrupted treatment with BLINCYTO. For clinical management of elevated liver enzymes, see section 4.4.Leukoencephalopathy including progressive multifocal leukoencephalopathyLeukoencephalopathy has been reported. Patients with brain MRI/CT findings consistent with leukoencephalopathy experienced concurrent serious adverse events including confusional state, tremor, cognitive disorder, encephalopathy, and convulsion. Although there is a potential for the development of progressive multifocal leukoencephalopathy (PML), no case of PML has been reported in the pivotal study.Paediatric populationThere is limited experience in paediatric patients. BLINCYTO has been eva luated in paediatric patients with relapsed or refractory B-precursor ALL in a phase I/II dose escalation/eva luation study. At a dose higher than the recommended dose for adult patients, a case of fatal cardiac failure occurred in the setting of life-threatening cytokine release syndrome (CRS) and tumour lysis syndrome (TLS), see section 4.4.Other special populationsThere is limited experience with BLINCYTO in patients ≥ 75 years of age. Generally, safety was similar between elderly patients (≥ 65 years of age) and patients less than 65 years of age treated with BLINCYTO. However, elderly patients may be more susceptible to serious neurologic events such as cognitive disorder, encephalopathy and confusion.The safety of BLINCYTO has not been studied in patients with severe renal impairment.ImmunogenicityIn the pivotal clinical study (N = 189), less than 1.4% of patients treated with blinatumomab tested positive for binding and neutralising anti-blinatumomab antibodies. All patients who tested positive for binding antibodies also tested positive for neutralising anti-blinatumomab antibodies. Anti-blinatumomab antibody formation might affect pharmacokinetics of blinatumomab.If formation of anti-blinatumomab antibodies with a clinically significant effect is suspected, contact the Marketing Authorisation Holder to discuss antibody testing. Contact details are provided in section 6 of the package leaflet.Reporting of suspected adverse reactionsReporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via:United KingdomYellow Card SchemeWebsite: www.mhra.gov.uk/yellowcardIrelandHPRA PharmacovigilanceEarlsfort TerraceIRL - Dublin 2Tel: +353 1 6764971Fax: +353 1 6762517Website: www.hpra.iee-mail: medsafety@hpra.ie4.9 OverdoseOverdoses have been observed including one patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration. Overdoses resulted in adverse reactions which were consistent with the reactions observed at the recommended therapeutic dose and included fever, tremors, and headache. In the event of overdose, the infusion should be temporarily interrupted and patients should be monitored. Reinitiation of BLINCYTO at the correct therapeutic dose should be considered when all toxicities have resolved and no earlier than 12 hours after interruption of the infusion (see section 4.2).5. Pharmacological properties5.1 Pharmacodynamic propertiesPharmacotherapeutic group: Antineoplastic agents, other Antineoplastic agents, ATC code: L01XC19.Mechanism of actionBlinatumomab is a bispecific T-cell engager antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T-cells. It activates endogenous T-cells by connecting CD3 in the T-cell receptor (TCR) complex with CD19 on benign and malignant B-cells. The anti-tumour activity of blinatumomab immunotherapy is not dependent on T-cells bearing a specific TCR or on peptide antigens presented by cancer cells, but is polyclonal in nature and independent of human leukocyte antigen (HLA) molecules on target cells. Blinatumomab mediates the formation of a cytolytic synapse between the T-cell and the tumour cell, releasing proteolytic enzymes to kill both proliferating and resting target cells. Blinatumomab is associated with transient upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T-cells, and results in elimination of CD19+ cells.Pharmacodynamic effectsConsistent immune-pharmacodynamic responses were observed in patients studied. During the continuous intravenous infusion over 4 weeks, the pharmacodynamic response was characterised by T-cell activation and initial redistribution, rapid peripheral B-cell depletion, and transient cytokine elevation.Peripheral T-cell redistribution (i.e., T-cell adhesion to blood vessel endothelium and/or transmigration into tissue) occurred after start of blinatumomab infusion or dose escalation. T-cell counts initially declined within 1 to 2 days and then returned to baseline levels within 7 to 14 days in the majority of patients. Increase of T-cell counts above baseline (T-cell expansion) was observed in few patients.Peripheral B-cell counts decreased rapidly to an undetectable level during treatment at doses ≥ 5 mcg/m2/day or ≥ 9 mcg/day in the majority of patients. No recovery of peripheral B-cell counts was observed during the 2-week treatment-free period between treatment cycles. Incomplete depletion of B-cells occurred at doses of 0.5 mcg/m2/day and 1.5 mcg/m2/day and in a few non-responders at higher doses.Cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α and IFN-γ were measured and, IL-6, IL-10 and IFN-γ were most elevated. Transient elevation of cytokines was observed in the first two days following start of blinatumomab infusion. The elevated cytokine levels returned to baseline within 24 to 48 hours during the infusion. In subsequent treatment cycles, cytokine elevation occurred in fewer patients with lesser intensity compared to the initial 48 hours of the first treatment cycle.Clinical efficacy and safetyA total of 225 patients aged ≥ 18 years of age with relapsed or refractory B-precursor ALL were exposed to BLINCYTO during clinical trials.BLINCYTO was eva luated in an open-label, multicentre, single-arm phase II study of 189 patients. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-precursor ALL (relapsed with first remission duration of ≤ 12 months in first salvage, or relapsed or refractory after first salvage therapy, or relapsed within 12 months of allogeneic HSCT, and had ≥ 10% blasts in bone marrow).Patients were premedicated with a mandatory cerebrospinal fluid prophylaxis consisting of an intrathecal regimen according to institutional or national guidelines within 1 week prior to start of blinatumomab treatment. BLINCYTO was administered as a continuous intravenous infusion. In the first cycle, the initial dose was 9 mcg/day for week 1, then 28 mcg/day for the remaining 3 weeks. The target dose of 28 mcg/day was administered in cycle 2 and subsequent cycles starting on day 1 of each cycle. Dose adjustment was possible in the case of adverse events. The treated population included 189 patients who received at least 1 infusion of BLINCYTO; the mean number of cycles per patient was 1.6. Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO. Among treated patients, the median age was 39 years (range: 18 to 79 years, including 25 patients ≥ 65 years of age), 64 of 189 (33.9%) had undergone HSCT prior to receiving BLINCYTO and 32 of 189 (16.9%) had received more than 2 prior salvage therapies.The primary endpoint was the complete remission/complete remission with partial haematological recovery (CR/CRh*) rate within 2 cycles of treatment with BLINCYTO. Eighty-one of 189 (42.9%) patients achieved CR/CRh* within the first 2 treatment cycles with the majority of responses (64 of 81) occurring within 1 cycle of treatment. In the elderly population (≥ 65 years of age) 11 of 25 patients (44.0%) achieved CR/CRh* within the first 2 treatment cycles (see section 4.8 for safety in elderly). Four patients achieved CR during consolidation cycles, resulting in a cumulative CR rate of 35.4% (67/189; 95% CI: 28.6% - 42.7%). Thirty-two of 189 (17%) patients underwent allogeneic HSCT in CR/CRh* induced with BLINCYTO (see table 1).Table 1. Efficacy results in patients ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL)In a prespecified exploratory analysis, 60 of 73 MRD eva luable patients with CR/CRh* (82.2%) also had a MRD response (defined as MRD by PCR < 1 x 10-4).Patients with prior allogeneic HSCT had similar response rates to those without prior HSCT, older patients had similar response rates to younger patients, and no substantial difference was observed in remission rates based on the number of lines of prior salvage treatment.In patients with non-CNS/non-testes extramedullary disease (defined as at least 1 lesion ≥ 1.5 cm) at screening (N = 8/189) clinical response rates (25% [95% CI 3.2-65.1] were lower compared with patients with no evidence of extramedullary disease (N = 181, 43.6% [95%CI 36.3 - 51.2]) (see figure 1).Patients with the highest tumour burden as measured by the percentage of bone marrow blast cells at baseline (≥ 90%) still had a clinically meaningful response with a CR/CRh* rate of 21.6% (CI 12.9 – 32.7) (see figure 1). Patients with low tumour burden (< 50%) responded best to blinatumomab treatment with CR/CRh* rate of 72.9% (CI 59.7 – 83.6).Figure 1. Forest plot of CR/CRh* rate during the first two cycles for study MT103-211 (primary analysis set)In an open-label, multicentre, dose-escalation phase II study of 36 patients (≥ 18 years of age with B-precursor ALL relapsed after at least induction and consolidation or having refractory disease with > 5% blasts in bone marrow, ECOG performance status ≤ 2, life expectancy of ≥ 12 weeks, who did not have autologous hematopoietic stem cell transplantation (HSCT) within 6 weeks prior to start of blinatumomab treatment, allogeneic HSCT within 3 months prior to start of blinatumomab treatment, or previous treatment with blinatumomab), the safety and efficacy of blinatumomab were eva luated. Fifteen of 36 (41.7%) patients had undergone allogeneic HSCT prior to receiving BLINCYTO. The CR/CRh* rate was 69.4% (25 out of 36 patients: 15 [41.7%; 95% CI: 25.5% - 59.2%] CR; 10 [27.8%; 95% CI: 14.2% - 45.2%] CRh*). In the elderly population (≥ 65 years of age) 4 of 5 patients (80.0%) achieved CR/CRh* within 2 treatment cycles (see section 4.8 for safety in elderly). Twenty-two of 25 (88%) patients with haematologic complete remission also had minimal residual disease (MRD) responses (defined as MRD by PCR < 1 x 10-4). The median duration of remission was 8.9 months, and the median relapse-free survival (RFS) was 7.6 months. The median overall survival (OS) was 9.8 months.There is limited data in patients with late first relapse of B-precursor ALL defined as a relapse occurring more than 12 months after first remission or more than 12 months after HSCT in the first remission. In clinical studies, 88.9% (8/9) of patients with late first relapse as defined in the individual studies achieved CR/CRh* within the first 2 treatment cycles with 62.5% (6/9) achieving MRD response and 37.5 % (3/9) undergoing allogeneic HSCT after treatment with blinatumomab. The median overall survival (OS) was 17.7 months (CI 3.1 – not estimable).Paediatric populationThere is limited experience in paediatric patients, see section 4.8.The European Medicines Agency has deferred the obligation to submit the results of studies with BLINCYTO in children from 1 month to less than 18 years of age with acute lymphoblastic leukaemia (see section 4.2 for information on paediatric use).This medicinal product has been authorised under a so called 'conditional approval' scheme. This means that further evidence on this medicinal product is awaited. The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.5.2 Pharmacokinetic propertiesThe pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 90 mcg/m2/day (approximately equivalent to 9-162 mcg/day) in adult patients. Following continuous intravenous infusion, the steady state serum concentration (Css) was achieved within a day and remained stable over time. The increase in mean Css values was approximately proportional to the dose in the range tested. At the clinical doses of 9 mcg/day and 28 mcg/day for the treatment of relapsed/refractory ALL, the mean (SD) Css was 211 (258) pg/mL and 621 (502) pg/mL, respectively.DistributionThe estimated mean (SD) volume of distribution based on terminal phase (Vz) was 4.52 (2.89) L with the continuous intravenous infusion of blinatumomab.BiotransformationThe metabolic pathway of blinatumomab has not been characterised. Like other protein therapeutics, blinatumomab is expected to be degraded into small peptides and amino acids via catabolic pathways.EliminationThe estimated mean (SD) systemic clearance with continuous intravenous infusion in patients receiving blinatumomab in clinical studies was 2.92 (2.83) L/hour. The mean (SD) half-life was 2.11 (1.42) hours. Negligible amounts of blinatumomab were excreted in the urine at the tested clinical doses.Body weight, body surface area, gender and ageA population pharmacokinetic analysis was performed to eva luate the effects of demographic characteristics on blinatumomab pharmacokinetics. Results suggest that age (18 to 80 years), gender, body weight (44 to 134 kg), and body surface area (1.39 to 2.57) do not influence the pharmacokinetics of blinatumomab. There is very limited experience with blinatumomab in adults weighing less than 45 kg.Renal impairmentNo formal pharmacokinetic studies of blinatumomab have been conducted in patients with renal impairment.Pharmacokinetic analyses showed an approximately 2-fold difference in mean blinatumomab clearance values between patients with moderate renal dysfunction and normal renal function. However high inter-patient variability was discerned (CV% up to 95.6%), and clearance values in renal impaired patients were essentially within the range observed in patients with normal renal function, no clinically meaningful impact of renal function on clinical outcomes is expected.Hepatic impairmentNo formal pharmacokinetic studies of blinatumomab have been conducted in patients with hepatic impairment. Baseline ALT and AST levels were used to assess the effect of hepatic impairment on the clearance of blinatumomab. Population pharmacokinetic analysis suggested that there was no association between ALT or AST levels and the clearance of blinatumomab.Paediatric populationThere is limited experience in paediatric patients.5.3 Preclinical safety dataRepeat-dose toxicity studies conducted with blinatumomab and the murine surrogate revealed the expected pharmacologic effects (including release of cytokines, decreases in leukocyte counts, depletion of B-cells, decreases in T-cells, decreased cellularity in lymphoid tissues). These changes reversed after cessation of treatment.Reproductive toxicity studies have not been conducted with blinatumomab. In an embryo-foetal developmental toxicity study performed in mice, the murine surrogate crossed the placenta to a limited extent (foetal-to-maternal serum concentration ratio < 1%) and did not induce embryo-foetal toxicity or teratogenicity. The expected depletions of B- and T-cells were observed in the pregnant mice but haematological effects were not assessed in foetuses. No studies have been conducted to eva luate treatment-related effects on fertility. There were no effects on male or female reproductive organs in toxicity studies with the murine surrogate.6. Pharmaceutical particulars6.1 List of excipientsPowderCitric acid monohydrate (E330)Trehalose dihydrateLysine hydrochloridePolysorbate 80Sodium hydroxide (for pH-adjustment)Solution (stabiliser)Citric acid monohydrate (E330)Lysine hydrochloridePolysorbate 80Sodium hydroxide (for pH-adjustment)Water for injections6.2 IncompatibilitiesThis medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.6.3 Shelf lifeUnopened vials3 yearsReconstituted solutionChemical and physical in-use stability has been demonstrated for 24 hours at 2°C – 8°C or 4 hours at or below 27°C.From a microbiological point of view, unless the method of reconstituting precludes the risks of microbial contamination, the reconstituted solution should be diluted immediately. If not diluted immediately, in-use storage times and conditions are the responsibility of the user.Diluted solution (prepared infusion bag)Chemical and physical in-use stability has been demonstrated for 10 days at 2°C – 8°C or 96 hours at or below 27°C.From a microbiological point of view, the prepared infusion bags should be used immediately.If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C – 8°C, unless dilution has taken place in controlled and validated aseptic conditions.6.4 Special precautions for storageStore and transport refrigerated (2°C – 8°C).Do not freeze.Store the vials in the original package in order to protect from light.For storage conditions after reconstitution and dilution of the medicinal product, see section 6.3.6.5 Nature and contents of containerEach BLINCYTO pack contains 1 vial of powder for concentrate for solution for infusion and 1 vial of solution (stabiliser):• 38.5 micrograms blinatumomab powder in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap and• 10 mL solution in a vial (type I glass) with a stopper (elastomeric rubber), seal (aluminium) and a flip off cap.6.6 Special precautions for disposal and other handlingAseptic preparationAseptic handling must be ensured when preparing the infusion. Preparation of BLINCYTO should be:- performed under aseptic conditions by trained personnel in accordance with good practice rules especially with respect to the aseptic preparation of parenteral products.- prepared in a laminar flow hood or biological safety cabinet using standard precautions for the safe handling of intravenous agents.It is very important that the instructions for preparation and administration provided in this section are strictly followed to minimise medication errors (including underdose and overdose).Special instructions to support accurate preparation• A solution (stabiliser) is provided inside the BLINCYTO package and is used to coat the pre-filled infusion bag prior to addition of reconstituted BLINCYTO. Do not use this solution (stabiliser)for reconstitution of BLINCYTO powder for concentrate.• The entire volume of the reconstituted and diluted BLINCYTO will be more than the volume to be administered to the patient (240 mL). This is to account for intravenous infusion line loss and to assure that the patient will receive the full dose of BLINCYTO.• When preparing an infusion bag, remove all air from infusion bag. This is particularly important when using an ambulatory infusion pump.• Use the specific volumes described in the reconstitution and dilution instructions below to minimise errors in calculation.Other instructions• BLINCYTO is compatible with polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.• Pump specifications: The infusion pump to administer BLINCYTO solution for infusion should be programmable, lockable and have an alarm. Elastomeric pumps should not be used.• Any unused medicinal product or waste material should be disposed of in accordance with local requirements.Preparation of the solution for infusionSpecific reconstitution and dilution instructions are provided for each dose and infusion time. Verify the prescribed dose and infusion time of BLINCYTO and identify the appropriate dosing preparation section listed below. Follow the steps for reconstituting BLINCYTO and preparing the infusion bag.a) for 9 mcg/day infused over 24 hours at a rate of 10 mL/hb) for 9 mcg/day infused over 48 hours at a rate of 5 mL/hc) for 9 mcg/day infused over 72 hours at a rate of 3.3 mL/hd) for 9 mcg/day infused over 96 hours at a rate of 2.5 mL/he) for 28 mcg/day infused over 24 hours at a rate of 10 mL/hf) for 28 mcg/day infused over 48 hours at a rate of 5 mL/hg) for 28 mcg/day infused over 72 hours at a rate of 3.3 mL/hh) for 28 mcg/day infused over 96 hours at a rate of 2.5 mL/hBefore preparation, ensure you have the following supplies ready:These supplies are also required, but not included in the package• Sterile single-use disposable syringes• 21-23 gauge needle(s) (recommended)• Water for injections• Infusion bag with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection;o To minimise the number of aseptic transfers, use a 250 mL pre-filled infusion bag. BLINCYTO dose calculations are based on a usual overfill volume of 265 to 275 mL sodium chloride 9 mg/mL (0.9%) solution for injection.o Use only polyolefin, PVC non-di-ethylhexylphthalate (non-DEHP), or ethyl vinyl acetate (EVA) infusion bags/pump cassettes.• Polyolefin, PVC non-DEHP, or EVA intravenous tubing with a sterile, non-pyrogenic, low protein-binding 0.2 micrometre in-line filtero Ensure that the tubing is compatible with the infusion pump.a) Preparation of BLINCYTO 9 mcg/day infused over 24 hours at a rate of 10 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 0.83 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.b) Preparation of BLINCYTO 9 mcg/day infused over 48 hours at a rate of 5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 1.7 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.c) Preparation of BLINCYTO 9 mcg/day infused over 72 hours at a rate of 3.3 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 2.5 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.d) Preparation of BLINCYTO 9 mcg/day infused over 96 hours at a rate of 2.5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use two vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 3.5 mL of reconstituted BLINCYTO into the infusion bag (2.0 mL from one vial and the remaining 1.5 mL from the second vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.e) Preparation of BLINCYTO 28 mcg/day infused over 24 hours at a rate of 10 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Using a syringe, reconstitute one vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 2.6 mL of reconstituted BLINCYTO into the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.f) Preparation of BLINCYTO 28 mcg/day infused over 48 hours at a rate of 5 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use two vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 5.2 mL of reconstituted BLINCYTO into the infusion bag (2.7 mL from one vial and the remaining 2.5 mL from the second vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.g) Preparation of BLINCYTO 28 mcg/day infused over 72 hours at a rate of 3.3 mL/h1. Use an infusion bag pre-filled with 250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use three vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 8 mL of reconstituted BLINCYTO into the infusion bag (2.8 mL from each of the first two vials and the remaining 2.4 mL from the third vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.h) Preparation of BLINCYTO 28 mcg/day infused over 96 hours at a rate of 2.5 mL/h1. Use an infusion bag pre-filled with250 mL sodium chloride 9 mg/mL (0.9%) solution for injection that usually contains a total volume of 265 to 275 mL.2. To coat the infusion bag, using a syringe, aseptically transfer 5.5 mL of the solution (stabiliser) to the infusion bag. Gently mix the contents of the bag to avoid foaming. Discard the remaining solution (stabiliser) vial.3. Use four vials of BLINCYTO powder for concentrate. Using a syringe, reconstitute each vial of BLINCYTO powder for concentrate using 3 mL of water for injections. Direct the water for injections toward the side of the vial during reconstitution. Gently swirl contents to avoid excess foaming. Do not shake.• Do not reconstitute BLINCYTO powder for concentrate with the solution (stabiliser).• The addition of water for injections to the powder for concentrate results in a total volume of 3.08 mL for a final BLINCYTO concentration of 12.5 mcg/mL.4. Visually inspect the reconstituted solution for particulate matter and discolouration during reconstitution and prior to infusion. The resulting solution should be clear to slightly opalescent, colourless to slightly yellow. Do not use if the solution is cloudy or has precipitated.5. Using a syringe, aseptically transfer 10.7 mL of reconstituted BLINCYTO into the infusion bag (2.8 mL from each of the first three vials and the remaining 2.3 mL from the fourth vial). Gently mix the contents of the bag to avoid foaming. Discard any remaining BLINCYTO reconstituted solution.6. Under aseptic conditions, attach the intravenous tubing to the infusion bag with the sterile 0.2 micron in-line filter.7. Remove air from the infusion bag and prime the intravenous infusion line only with the prepared solution for infusion. Do not prime with sodium chloride 9 mg/mL (0.9%) solution for injection.8. Store at 2°C – 8°C if not used immediately.For instructions on administration, see section 4.2.7. Marketing authorisation holderAmgen Europe B.V.Minervum 70614817 ZK BredaThe Netherlands8. Marketing authorisation number(s)EU/1/15/1047/0019. Date of first authorisation/renewal of the authorisationDate of first authorisation: 23 November 201510. Date of revision of the textNovember 2015Detailed information on this medicinal product is available on the website of the European Medicines Agency https://www.ema.europa.eu
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博纳吐单抗冻干粉注射剂Blincyto Injection 38.5mcg(Blinatumomab)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博纳吐单抗冻干粉注射剂Blincyto Injection 38.5mcg(Blinatumomab)


