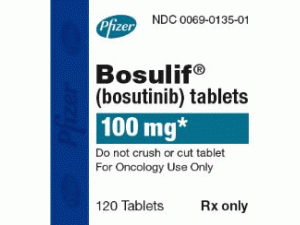
博舒替尼片BOSULIF Tablets 100mg(BOSUTINIB)
 药店国别:
产地国家:美国
处方药:是
所属类别: 100毫克/片 120片/瓶
包装规格: 100毫克/片 120片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:BOSULIF 100MG/Tablets 120Tablets/bottle
原产地英文药品名:BOSUTINIB
中文参考商品译名:BOSULIF片 100毫克/片 120片/瓶
中文参考药品译名:伯舒替尼
曾用名:波舒替尼
简介:近日,美国食品和药物管理局(FDA)批准补充新药申请(sNDA,扩大Bosulif(bosutinib)的适应症,包括新诊断的慢性期费城染色体阳性慢性粒细胞白血病患者(Ph +)CML)。批准日期:2012年 公司:辉瑞公司BOSULIF(博舒替尼[bosutinib])片剂,口服使用美国最初批准:2012年最近的重大变化适应症和用法,新诊断的慢性期Ph + CML:12/2017
作用机制
Bosutinib是TKI。Bosutinib抑制促进CML的BCR-ABL激酶; 它也是Src家族激酶的抑制剂,包括Src,Lyn和Hck。Bosutinib抑制了在鼠骨髓细胞系中表达的18种伊马替尼耐药形式的BCR-ABL激酶中的16种。 Bosutinib不抑制T315I和V299L突变细胞。
适应症和用法
BOSULIF是一种激酶抑制剂,适用于治疗成人患者新诊断的慢性期Ph+慢性粒细胞白血病(CML)。该指示基于分子和细胞遗传学应答率在加速批准下获得批准。持续批准该适应症可能取决于正在进行的长期随访试验中的临床获益的验证和确认。慢性,加速或急变期Ph+CML,对先前治疗具有耐药性或不耐受性。
剂量和给药
新诊断的慢性期Ph+CML:每日口服400毫克,食物一次。慢性,加速或爆炸期Ph+CML对先前治疗具有耐药性或不耐受性:每日一次口服500毫克食物。对于未达到完全血液学,细胞遗传学或分子反应并且没有3级或更高级不良反应的患者,考虑每日一次增加100mg剂量至每日最多600mg的剂量增加。调整剂量以防止毒性和器官损害剂量形式和强度片剂:100mg,400mg和500mg。
禁忌症
对BOSULIF过敏。
警告和注意事项
胃肠道毒性:根据需要进行监测和管理。扣留,剂量减少或停止使用BOSULIF。骨髓抑制:监测血液计数并根据需要进行管理。肝脏毒性:在前3个月至少每月监测一次肝酶,并根据需要进行监测。扣留,剂量减少或停止使用BOSULIF。液体保留:监测患者并使用标准治疗方法进行管理。扣留,剂量减少或停止使用BOSULIF。肾毒性:在基线和BOSULIF治疗期间监测患者的肾功能。胚胎-胎儿毒性:BOSULIF可导致胎儿伤害。告知患有胎儿潜在风险并使用有效避孕措施的患者。
不良反应
新诊断的CML患者最常见的不良反应(发生率≥20%)是腹泻,恶心,血小板减少,皮疹,丙氨酸氨基转移酶升高,腹痛,天冬氨酸氨基转移酶升高。CML患者对既往治疗耐药或不耐受(发病率≥20%)的最常见不良反应是腹泻,恶心,腹痛,皮疹,血小板减少,呕吐,贫血,疲劳,发热,咳嗽,头痛,丙氨酸氨基转移酶,以及浮肿。
药物相互作用
强效和中等CYP3A抑制剂:避免与BOSULIF同时使用。强CYP3A诱导剂:避免与BOSULIF同时使用。质子泵抑制剂:使用短效抗酸剂或H2阻滞剂作为质子泵抑制剂的替代品。
用于特定人群哺乳期:
建议女性不要母乳喂养。
包装提供/存储和处理提供
BOSULIF(bosutinib)片剂以3种强度口服给药:100mg黄色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“100”压印;一片400毫克橙色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“400”压印; 500毫克红色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“500”压印。 BOSULIF(bosutinib)片剂有以下包装配置(表11):每瓶120片,100mg NDC:0069-0135-01每瓶30片, 400mg NDC:0069-0193-01每瓶30片 500mg NDC:0069-0136-01缩写:NDC =国家药物代码。
存储
储存在20°C至25°C(68°F至77°F);允许偏移15°C至30°C(59°F至86°F)[见USP受控室温]。处理和处置应考虑正确处理抗癌药物的程序。应避免接触或处理压碎或破碎的药片。任何未使用的产品或废料应根据当地要求或药物回收计划进行处置。
博舒替尼片英文版说明书
BOSULIF is a prescription medicine used to treat adults who have a type of leukemia called Philadelphia chromosome–positive chronic myelogenous leukemia (Ph+ CML) who no longer benefit from or did not tolerate other treatment.Important Safety Information for patientsDo not take BOSULIF if you are allergic to bosutinib or any of the ingredients in BOSULIF.BOSULIF may cause serious side effects, including:Stomach problems. BOSULIF may cause stomach (abdomen) pain, nausea, diarrhea, or vomiting. Tell your doctor about any stomach problemsLow blood cell counts. BOSULIF may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia) and low white blood cell counts (neutropenia). Your doctor should do blood tests to check your blood cell counts regularly during your treatment with BOSULIF. Call your doctor right away if you have unexpected bleeding or bruising, blood in your urine or stools, fever, or any signs of an infectionLiver problems. BOSULIF may cause liver problems. Your doctor should do blood tests to check your liver function regularly during your treatment with BOSULIF. Call your doctor right away if your skin or the white part of your eyes turns yellow (jaundice) or you have dark “tea color” urineYour body may hold too much fluid (fluid retention). Fluid may build up in the lining of your lungs, the sac around your heart, or your stomach cavity. Call your doctor right away if you get any of the following symptoms during your treatment with BOSULIF:– shortness of breath and cough– chest pain– swelling in your hands, ankles, or feet– swelling all over your body– weight gainThe most common side effects of BOSULIF include:– diarrhea– nausea– low blood cell counts– vomiting– stomach pain– rash– fever– tiredness or weaknessTell your doctor right away if you get respiratory tract infections, loss of appetite, headache, dizziness, back pain, joint pain, or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction.Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of BOSULIF. For more information, ask your doctor or pharmacist.Tell your doctor about the medicines you take, including prescription medicines, non-prescription medicines, vitamins, and herbal supplements. BOSULIF and certain other medicines can affect each other.Before you take BOSULIF, tell your doctor if you:have liver problemshave heart problemshave kidney problemshave any other medical conditionsare pregnant or plan to become pregnant. BOSULIF can harm your unborn baby. You should not become pregnant while taking BOSULIF. Tell your doctor right away if you become pregnant while taking BOSULIFare a woman who may become pregnant. Use effective contraception (birth control) during and for at least 30 days after completing treatment with BOSULIF. Talk to your doctor about forms of birth controlare breastfeeding or plan to breastfeed. It is not known if BOSULIF passes into your breast milk or if it can harm your baby. You and your doctor should decide if you will take BOSULIF or breastfeed. You should not do both
药店国别:
产地国家:美国
处方药:是
所属类别: 100毫克/片 120片/瓶
包装规格: 100毫克/片 120片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:BOSULIF 100MG/Tablets 120Tablets/bottle
原产地英文药品名:BOSUTINIB
中文参考商品译名:BOSULIF片 100毫克/片 120片/瓶
中文参考药品译名:伯舒替尼
曾用名:波舒替尼
简介:近日,美国食品和药物管理局(FDA)批准补充新药申请(sNDA,扩大Bosulif(bosutinib)的适应症,包括新诊断的慢性期费城染色体阳性慢性粒细胞白血病患者(Ph +)CML)。批准日期:2012年 公司:辉瑞公司BOSULIF(博舒替尼[bosutinib])片剂,口服使用美国最初批准:2012年最近的重大变化适应症和用法,新诊断的慢性期Ph + CML:12/2017
作用机制
Bosutinib是TKI。Bosutinib抑制促进CML的BCR-ABL激酶; 它也是Src家族激酶的抑制剂,包括Src,Lyn和Hck。Bosutinib抑制了在鼠骨髓细胞系中表达的18种伊马替尼耐药形式的BCR-ABL激酶中的16种。 Bosutinib不抑制T315I和V299L突变细胞。
适应症和用法
BOSULIF是一种激酶抑制剂,适用于治疗成人患者新诊断的慢性期Ph+慢性粒细胞白血病(CML)。该指示基于分子和细胞遗传学应答率在加速批准下获得批准。持续批准该适应症可能取决于正在进行的长期随访试验中的临床获益的验证和确认。慢性,加速或急变期Ph+CML,对先前治疗具有耐药性或不耐受性。
剂量和给药
新诊断的慢性期Ph+CML:每日口服400毫克,食物一次。慢性,加速或爆炸期Ph+CML对先前治疗具有耐药性或不耐受性:每日一次口服500毫克食物。对于未达到完全血液学,细胞遗传学或分子反应并且没有3级或更高级不良反应的患者,考虑每日一次增加100mg剂量至每日最多600mg的剂量增加。调整剂量以防止毒性和器官损害剂量形式和强度片剂:100mg,400mg和500mg。
禁忌症
对BOSULIF过敏。
警告和注意事项
胃肠道毒性:根据需要进行监测和管理。扣留,剂量减少或停止使用BOSULIF。骨髓抑制:监测血液计数并根据需要进行管理。肝脏毒性:在前3个月至少每月监测一次肝酶,并根据需要进行监测。扣留,剂量减少或停止使用BOSULIF。液体保留:监测患者并使用标准治疗方法进行管理。扣留,剂量减少或停止使用BOSULIF。肾毒性:在基线和BOSULIF治疗期间监测患者的肾功能。胚胎-胎儿毒性:BOSULIF可导致胎儿伤害。告知患有胎儿潜在风险并使用有效避孕措施的患者。
不良反应
新诊断的CML患者最常见的不良反应(发生率≥20%)是腹泻,恶心,血小板减少,皮疹,丙氨酸氨基转移酶升高,腹痛,天冬氨酸氨基转移酶升高。CML患者对既往治疗耐药或不耐受(发病率≥20%)的最常见不良反应是腹泻,恶心,腹痛,皮疹,血小板减少,呕吐,贫血,疲劳,发热,咳嗽,头痛,丙氨酸氨基转移酶,以及浮肿。
药物相互作用
强效和中等CYP3A抑制剂:避免与BOSULIF同时使用。强CYP3A诱导剂:避免与BOSULIF同时使用。质子泵抑制剂:使用短效抗酸剂或H2阻滞剂作为质子泵抑制剂的替代品。
用于特定人群哺乳期:
建议女性不要母乳喂养。
包装提供/存储和处理提供
BOSULIF(bosutinib)片剂以3种强度口服给药:100mg黄色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“100”压印;一片400毫克橙色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“400”压印; 500毫克红色,椭圆形,双凸面,薄膜包衣片,一面用“Pfizer”压印,另一面用“500”压印。 BOSULIF(bosutinib)片剂有以下包装配置(表11):每瓶120片,100mg NDC:0069-0135-01每瓶30片, 400mg NDC:0069-0193-01每瓶30片 500mg NDC:0069-0136-01缩写:NDC =国家药物代码。
存储
储存在20°C至25°C(68°F至77°F);允许偏移15°C至30°C(59°F至86°F)[见USP受控室温]。处理和处置应考虑正确处理抗癌药物的程序。应避免接触或处理压碎或破碎的药片。任何未使用的产品或废料应根据当地要求或药物回收计划进行处置。
博舒替尼片英文版说明书
BOSULIF is a prescription medicine used to treat adults who have a type of leukemia called Philadelphia chromosome–positive chronic myelogenous leukemia (Ph+ CML) who no longer benefit from or did not tolerate other treatment.Important Safety Information for patientsDo not take BOSULIF if you are allergic to bosutinib or any of the ingredients in BOSULIF.BOSULIF may cause serious side effects, including:Stomach problems. BOSULIF may cause stomach (abdomen) pain, nausea, diarrhea, or vomiting. Tell your doctor about any stomach problemsLow blood cell counts. BOSULIF may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia) and low white blood cell counts (neutropenia). Your doctor should do blood tests to check your blood cell counts regularly during your treatment with BOSULIF. Call your doctor right away if you have unexpected bleeding or bruising, blood in your urine or stools, fever, or any signs of an infectionLiver problems. BOSULIF may cause liver problems. Your doctor should do blood tests to check your liver function regularly during your treatment with BOSULIF. Call your doctor right away if your skin or the white part of your eyes turns yellow (jaundice) or you have dark “tea color” urineYour body may hold too much fluid (fluid retention). Fluid may build up in the lining of your lungs, the sac around your heart, or your stomach cavity. Call your doctor right away if you get any of the following symptoms during your treatment with BOSULIF:– shortness of breath and cough– chest pain– swelling in your hands, ankles, or feet– swelling all over your body– weight gainThe most common side effects of BOSULIF include:– diarrhea– nausea– low blood cell counts– vomiting– stomach pain– rash– fever– tiredness or weaknessTell your doctor right away if you get respiratory tract infections, loss of appetite, headache, dizziness, back pain, joint pain, or itching while taking BOSULIF. These may be symptoms of a severe allergic reaction.Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of BOSULIF. For more information, ask your doctor or pharmacist.Tell your doctor about the medicines you take, including prescription medicines, non-prescription medicines, vitamins, and herbal supplements. BOSULIF and certain other medicines can affect each other.Before you take BOSULIF, tell your doctor if you:have liver problemshave heart problemshave kidney problemshave any other medical conditionsare pregnant or plan to become pregnant. BOSULIF can harm your unborn baby. You should not become pregnant while taking BOSULIF. Tell your doctor right away if you become pregnant while taking BOSULIFare a woman who may become pregnant. Use effective contraception (birth control) during and for at least 30 days after completing treatment with BOSULIF. Talk to your doctor about forms of birth controlare breastfeeding or plan to breastfeed. It is not known if BOSULIF passes into your breast milk or if it can harm your baby. You and your doctor should decide if you will take BOSULIF or breastfeed. You should not do both
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博舒替尼片BOSULIF Tablets 100mg(BOSUTINIB)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博舒替尼片BOSULIF Tablets 100mg(BOSUTINIB)