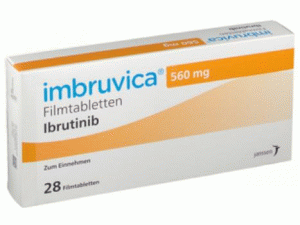
博舒替尼水合物BOSULIF Tablets 100mg
 药店国别:
产地国家:日本
处方药:是
所属类别: 100毫克/片 50片/盒
包装规格: 100毫克/片 50片/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:Bosulif(ボシュリフ錠)100MG/tablets 50tablets/box
原产地英文药品名:Bosutinib Hydrate
中文参考商品译名:Bosulif(ボシュリフ錠)100毫克/片 50片/盒
中文参考药品译名:博舒替尼水合物
曾用名:
简介:英文药名:Bosulif(Bosutinib Hydrate Tablets)中文药名: 博舒替尼水合物/片剂生产厂家:辉瑞公司药物分类名称抗肿瘤剂/酪氨酸激酶抑制剂批准日期:2014年12月商標名Bosulif tablets一般名ボスチニブ水和物(Bosutinib Hydrate)化学名4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propyloxy]quinoline-3-carbonitrile monohydrate分子式C26H29Cl2N5O3・H2O分子量548.46構造式性状本品为白色至黄棕色粉末。本产品溶于二甲基亚砜,微溶于乙醇(99.5),难溶于乙腈,甲醇,难溶于水。分配系数(log D)3.1(pH 7.4,1-辛醇/水)
药效药理
1. 抗肿瘤作用Bostinib体外抑制BCR-ABL融合基因阳性人慢性粒细胞白血病来源的细胞系(KU 812,K 562,Meg-01,Lama 84和KCL 22)的增殖。 此外,通过施用bostinib皮下移植K562细胞系的裸鼠中观察到肿瘤生长抑制作用和存活的延长。
2. 作用机制Bostinib通过抑制Abl和Src酪氨酸激酶活性来抑制BCR-ABL融合基因阳性肿瘤的增殖。适应症慢性粒细胞白血病耐药或不耐受治疗前药物
用法与用量
成人,每天一次,每次口服500mg, 剂量可以根据患者的情况而增加或减少,但是可以每天增加到600mg一次。包装规格片剂100毫克:50片(PTP)
制造和销售(进口)
辉瑞公司
产品简介
波舒替尼(也称伯舒替尼,Bosutinib,SKI 606)由美国惠氏制药公司(Wyeth Pharmaceuticals)研制,是一种强效的蛋白激酶Src/Ab1双重抑制剂。波舒替尼(Bosutinib)于2012年9月4日FDA批准用于慢性、加速或急变期Ph+CML成年患者。商品名:bosulif。大部分CML患者患有被称为费城染色体的基因突变,这导致骨髓产生酪氨酸激酶。这种酶触发骨髓产生过多的畸形不健康的白细胞即粒细胞。粒细胞可以对抗感染。波舒替尼(Bosulif)通过阻断酪氨酸激酶刺激骨髓加速产生畸形不健康的粒细胞的信号而发挥作用。波舒替尼是一种每天只需给药一次的口服激酶抑制剂类药物,波舒替尼(bosutinib)是SRC和BCR ABL双重抑制剂,该药通过抑制Abl与Src信号传导通路而遏制肿瘤细胞的生长。在Ⅰ-Ⅱ期临床研究中,69位伊马替尼耐药的慢性髓细胞白血病(chronic myelogenous leukemia, CML)或ph+ALL病人用伯舒替尼治疗后症状都得到了好转。
博舒替尼水合物英文版说明书
Acquired manufacturing and marketing authorization for "Bosliff ® tablet 100mg" for treatment of chronic myelogenous leukemia~ For chronic myelogenous leukemia, new treatment optionsPfizer Inc.(Headquarters: Shibuya-ku, Tokyo; President: Ichiro Umeda, hereinafter referred to as "Pfizer") announced on September 26(Fri) 2014, "Chronic myelogenous leukemia resistant or intolerable to pre-Manufacture of oral SRC/ABL tyrosine kinase inhibitor "Boschlaf®tablet 100mg"(generic name: Bosutibibu, hereinafter "Boshurif") with the efficacy and effect of the oral SRC /ABL tyrosine kinase inhibitor(hereinafter, "chronic myeloid leukemia"We obtained sales approval.Treatment with selective BCR-ABL tyrosine kinase imatinib, dasatinib or nilotinib, or allogeneic hematopoietic cell transplantation is currently being performed as the primary treatment option for CML.However, medical needs in the second and third and subsequent treatments are still high, as patients with sufficient first-line treatment can not obtain a therapeutic effect, or there are patients who need to be discontinued due to side effects.Boschliff is a candidate for CML patients with imatinib resistant or intolerable (second line) treatment in overseas and domestic clinical trials and also for CML patients with dasatinib or nilotinib resistant or intolerable (after tertiary therapy) Efficacy was recognized for patients, and tolerability was good.This approval is based on these test results.In addition, this drug was designated as a drug for rare diseases (Orphan drug) with the same efficacy and was under review as a priority review item.Overview of Bosliffproduct nameBosliff®tablet 100mg(BOSLIF®Tablets 100mg)common nameBosutinib (Bosutinib)Manufacturing salesSelling approval acquisition date September 26, 2014Manufacturing salesPfizer Japan IncIndicationChronic myelogenous leukemia resistant or intolerant to pretreatment drugsDosage regimenUsually, adults receive 500 mg once a day as a bostinib orally after meals. Although it may be increased or decreased according to the condition of the patient, it can be increased up to 600mg once a day.
药店国别:
产地国家:日本
处方药:是
所属类别: 100毫克/片 50片/盒
包装规格: 100毫克/片 50片/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:Bosulif(ボシュリフ錠)100MG/tablets 50tablets/box
原产地英文药品名:Bosutinib Hydrate
中文参考商品译名:Bosulif(ボシュリフ錠)100毫克/片 50片/盒
中文参考药品译名:博舒替尼水合物
曾用名:
简介:英文药名:Bosulif(Bosutinib Hydrate Tablets)中文药名: 博舒替尼水合物/片剂生产厂家:辉瑞公司药物分类名称抗肿瘤剂/酪氨酸激酶抑制剂批准日期:2014年12月商標名Bosulif tablets一般名ボスチニブ水和物(Bosutinib Hydrate)化学名4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propyloxy]quinoline-3-carbonitrile monohydrate分子式C26H29Cl2N5O3・H2O分子量548.46構造式性状本品为白色至黄棕色粉末。本产品溶于二甲基亚砜,微溶于乙醇(99.5),难溶于乙腈,甲醇,难溶于水。分配系数(log D)3.1(pH 7.4,1-辛醇/水)
药效药理
1. 抗肿瘤作用Bostinib体外抑制BCR-ABL融合基因阳性人慢性粒细胞白血病来源的细胞系(KU 812,K 562,Meg-01,Lama 84和KCL 22)的增殖。 此外,通过施用bostinib皮下移植K562细胞系的裸鼠中观察到肿瘤生长抑制作用和存活的延长。
2. 作用机制Bostinib通过抑制Abl和Src酪氨酸激酶活性来抑制BCR-ABL融合基因阳性肿瘤的增殖。适应症慢性粒细胞白血病耐药或不耐受治疗前药物
用法与用量
成人,每天一次,每次口服500mg, 剂量可以根据患者的情况而增加或减少,但是可以每天增加到600mg一次。包装规格片剂100毫克:50片(PTP)
制造和销售(进口)
辉瑞公司
产品简介
波舒替尼(也称伯舒替尼,Bosutinib,SKI 606)由美国惠氏制药公司(Wyeth Pharmaceuticals)研制,是一种强效的蛋白激酶Src/Ab1双重抑制剂。波舒替尼(Bosutinib)于2012年9月4日FDA批准用于慢性、加速或急变期Ph+CML成年患者。商品名:bosulif。大部分CML患者患有被称为费城染色体的基因突变,这导致骨髓产生酪氨酸激酶。这种酶触发骨髓产生过多的畸形不健康的白细胞即粒细胞。粒细胞可以对抗感染。波舒替尼(Bosulif)通过阻断酪氨酸激酶刺激骨髓加速产生畸形不健康的粒细胞的信号而发挥作用。波舒替尼是一种每天只需给药一次的口服激酶抑制剂类药物,波舒替尼(bosutinib)是SRC和BCR ABL双重抑制剂,该药通过抑制Abl与Src信号传导通路而遏制肿瘤细胞的生长。在Ⅰ-Ⅱ期临床研究中,69位伊马替尼耐药的慢性髓细胞白血病(chronic myelogenous leukemia, CML)或ph+ALL病人用伯舒替尼治疗后症状都得到了好转。
博舒替尼水合物英文版说明书
Acquired manufacturing and marketing authorization for "Bosliff ® tablet 100mg" for treatment of chronic myelogenous leukemia~ For chronic myelogenous leukemia, new treatment optionsPfizer Inc.(Headquarters: Shibuya-ku, Tokyo; President: Ichiro Umeda, hereinafter referred to as "Pfizer") announced on September 26(Fri) 2014, "Chronic myelogenous leukemia resistant or intolerable to pre-Manufacture of oral SRC/ABL tyrosine kinase inhibitor "Boschlaf®tablet 100mg"(generic name: Bosutibibu, hereinafter "Boshurif") with the efficacy and effect of the oral SRC /ABL tyrosine kinase inhibitor(hereinafter, "chronic myeloid leukemia"We obtained sales approval.Treatment with selective BCR-ABL tyrosine kinase imatinib, dasatinib or nilotinib, or allogeneic hematopoietic cell transplantation is currently being performed as the primary treatment option for CML.However, medical needs in the second and third and subsequent treatments are still high, as patients with sufficient first-line treatment can not obtain a therapeutic effect, or there are patients who need to be discontinued due to side effects.Boschliff is a candidate for CML patients with imatinib resistant or intolerable (second line) treatment in overseas and domestic clinical trials and also for CML patients with dasatinib or nilotinib resistant or intolerable (after tertiary therapy) Efficacy was recognized for patients, and tolerability was good.This approval is based on these test results.In addition, this drug was designated as a drug for rare diseases (Orphan drug) with the same efficacy and was under review as a priority review item.Overview of Bosliffproduct nameBosliff®tablet 100mg(BOSLIF®Tablets 100mg)common nameBosutinib (Bosutinib)Manufacturing salesSelling approval acquisition date September 26, 2014Manufacturing salesPfizer Japan IncIndicationChronic myelogenous leukemia resistant or intolerant to pretreatment drugsDosage regimenUsually, adults receive 500 mg once a day as a bostinib orally after meals. Although it may be increased or decreased according to the condition of the patient, it can be increased up to 600mg once a day.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博舒替尼水合物BOSULIF Tablets 100mg
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 博舒替尼水合物BOSULIF Tablets 100mg