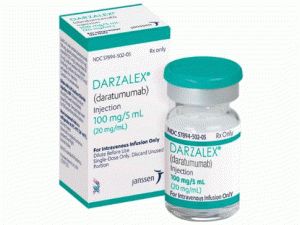
达雷木单抗注射溶液DARZALEX Injection 400mg/2ml(daratumumab)
 药店国别:
产地国家:美国
处方药:是
所属类别: 400毫克/20毫升(20毫升)/瓶
包装规格: 400毫克/20毫升(20毫升)/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Janssen Biotech, Inc.
原产地英文商品名:Darzalex 400mg/20nl(20ml)/Vial
原产地英文药品名:daratumumab
中文参考商品译名:Darzalex注射剂 400毫克/20毫升(20毫升)/瓶
中文参考药品译名:daratumumab
曾用名:
简介:近日,美国食品和药品监管局FDA授权加速批准Darzalex(daratumumab)治疗有多发性骨髓瘤患者曾接受至少三种以前治疗。Darzalex是被批准对治疗多发性骨髓瘤的第一个单克隆抗体。FDA的药物评价和研究中心中血液学和肿瘤室主任Richard Pazdur,M.D.说:“在癌症细胞表面上发现靶向蛋白已导致重要肿瘤治疗的进展,” “Darzalex提供对其他治疗已成为耐药的有多发性骨髓瘤患者另外治疗选择。”Darzalex注射,作为一种输注给予,是一种单克隆抗体通过帮助在免疫系统中某些细胞攻击癌细胞作用。批准日期:2015年11月16日 公司:强生制药DARZALEX(daratumumab)注射液,用于静脉注射最初的美国批准-2015年最近的重大变化适应症和用法。
作用机制
CD38是在造血细胞表面上表达的跨膜糖蛋白(48kDa),包括多发性骨髓瘤和其他细胞类型和组织,并且具有多种功能,例如受体介导的粘附,信号传导,以及环化酶和水解酶活性的调节。Daratumumab是一种IgG1κ人单克隆抗体(mAb),通过Fc介导的交联连接以及免疫介导的肿瘤细胞裂解通过补体依赖性细胞毒性(CDC),抗体依赖细胞,与CD38结合并抑制CD38表达肿瘤细胞的生长。介导的细胞毒性(ADCC)和抗体依赖性细胞吞噬作用(ADCP)。daratumumab减少了髓样来源抑制细胞(CD38+MDSCs),调节性T细胞(CD38+Tregs)和B细胞(CD38+Bregs)的子集。
适应症和用法
DARZALEX是一种CD38指导的溶细胞抗体,表明:与硼替佐米,美法仑和泼尼松联合用于治疗新诊断的多发性骨髓瘤患者,这些患者与来那度胺和地塞米松或硼替佐米和地塞米松联合用于自体干细胞移植,用于治疗患有多发性骨髓瘤的患者,至少接受过一次治疗,联合使用pomalidomide和地塞米松治疗多发性骨髓瘤患者至少接受过两次治疗,包括来那度胺和蛋白酶体抑制剂单药治疗,用于治疗多发性骨髓瘤患者,这些患者至少接受过三种治疗方法,包括蛋白酶抑制剂(PI)和免疫调节剂,或者是双重耐药剂PI和免疫调节剂。
剂量和给药
用皮质类固醇,退热药和抗组胺药预先给药。稀释并作为静脉输注给药。推荐剂量为16mg/kg实际体重。查看组合和时间表中使用的药物的完整处方信息。管理输液后的药物。
剂量形式和强度
注射:在单剂量小瓶中的100mg/5mL溶液。在单剂量小瓶中加入400mg/20mL溶液。
禁忌症
对daratumumab或制剂的任何组分有严重超敏反应史的患者。
警告和注意事项
输液反应:中断DARZALEX输液用于输液反应的严重程度。在过敏反应或危及生命的输液反应的情况下,永久停止输液,并提供适当的紧急护理。交叉匹配和红细胞抗体筛查的干扰:在开始治疗之前对患者进行分类和筛选。通知血库,患者已收到DARZALEX。中性粒细胞减少症:在治疗期间定期监测完整的血细胞计数。监测中性粒细胞减少症患者的感染迹象。可能需要Dosedelay来恢复中性粒细胞。血小板减少症:在治疗期间定期监测全血细胞计数。可能需要剂量延迟以允许血小板的恢复。
不良反应
最常报告的不良反应(发生率≥20%)为:输液反应,中性粒细胞减少,血小板减少,疲劳,恶心,腹泻,便秘,呕吐,肌肉痉挛,关节痛,背痛,发热,畏寒,头晕,失眠,咳嗽,呼吸困难,外周水肿,外周感觉神经病和上呼吸道感染。
包装提供/存储和处理提供
DARZALEX是一种无色至淡黄色,不含防腐剂的静脉输注溶液,提供:NDC 57894-502-05含有一个100mg/5mL单剂量小瓶NDC 57894-502-20含有一个400mg/20mL单剂量小瓶储存和稳定性储存在2ºC至8ºC(36ºF至46ºF)的冰箱中。不要冻结或摇晃。 避光。
达雷木单抗注射溶液英文版说明书
in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least one prior therapy.Daratumumab was previously granted accelerated approval in November 2015 as monotherapy for patients with multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent, or who are double refractory to a PI and an immunomodulatory agent.The current approval was based on two randomized, open-label trials in which daratumumab was added to standard therapies. The POLLUX trial (also known as MMY3003), demonstrated substantial improvement in progression-free survival (PFS) when daratumumab was added to lenalidomide and dexamethasone compared with lenalidomide and dexamethasone alone. The estimated median PFS had not been reached in the daratumumab arm and was 18.4 months in the control arm (HR=0.37; 95% CI: 0.27, 0.52; p<0.0001), representing a 63% reduction in the risk of disease progression or death in patients treated with daratumumab.Similar results were observed in the CASTOR trial (also known as MMY3004), which compared the combination of daratumumab, bortezomib, and dexamethasone with bortezomib and dexamethasone. The estimated median PFS was not reached in the daratumumab arm and was 7.2 months in the control arm (HR=0.39; 95% CI: 0.28, 0.53; p<0.0001), representing a 61% reduction in the risk of disease progression or death for patients treated with daratumumab.The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3003 were infusion reactions, diarrhea, nausea, fatigue, pyrexia, upper respiratory tract infection, muscle spasm, cough and dyspnea. The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3004 were infusion reactions, diarrhea, peripheral edema, upper respiratory tract infection, peripheral sensory Neutropenia and thrombocytopenia have been added to the Warnings and Precautions of the DARZALEX label.The recommended dose of daratumumab is 16 mg/kg intravenously (calculated on actual body weight).FDA granted daratumumab breakthrough therapy and orphan drug designation, as well as priority review
药店国别:
产地国家:美国
处方药:是
所属类别: 400毫克/20毫升(20毫升)/瓶
包装规格: 400毫克/20毫升(20毫升)/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Janssen Biotech, Inc.
原产地英文商品名:Darzalex 400mg/20nl(20ml)/Vial
原产地英文药品名:daratumumab
中文参考商品译名:Darzalex注射剂 400毫克/20毫升(20毫升)/瓶
中文参考药品译名:daratumumab
曾用名:
简介:近日,美国食品和药品监管局FDA授权加速批准Darzalex(daratumumab)治疗有多发性骨髓瘤患者曾接受至少三种以前治疗。Darzalex是被批准对治疗多发性骨髓瘤的第一个单克隆抗体。FDA的药物评价和研究中心中血液学和肿瘤室主任Richard Pazdur,M.D.说:“在癌症细胞表面上发现靶向蛋白已导致重要肿瘤治疗的进展,” “Darzalex提供对其他治疗已成为耐药的有多发性骨髓瘤患者另外治疗选择。”Darzalex注射,作为一种输注给予,是一种单克隆抗体通过帮助在免疫系统中某些细胞攻击癌细胞作用。批准日期:2015年11月16日 公司:强生制药DARZALEX(daratumumab)注射液,用于静脉注射最初的美国批准-2015年最近的重大变化适应症和用法。
作用机制
CD38是在造血细胞表面上表达的跨膜糖蛋白(48kDa),包括多发性骨髓瘤和其他细胞类型和组织,并且具有多种功能,例如受体介导的粘附,信号传导,以及环化酶和水解酶活性的调节。Daratumumab是一种IgG1κ人单克隆抗体(mAb),通过Fc介导的交联连接以及免疫介导的肿瘤细胞裂解通过补体依赖性细胞毒性(CDC),抗体依赖细胞,与CD38结合并抑制CD38表达肿瘤细胞的生长。介导的细胞毒性(ADCC)和抗体依赖性细胞吞噬作用(ADCP)。daratumumab减少了髓样来源抑制细胞(CD38+MDSCs),调节性T细胞(CD38+Tregs)和B细胞(CD38+Bregs)的子集。
适应症和用法
DARZALEX是一种CD38指导的溶细胞抗体,表明:与硼替佐米,美法仑和泼尼松联合用于治疗新诊断的多发性骨髓瘤患者,这些患者与来那度胺和地塞米松或硼替佐米和地塞米松联合用于自体干细胞移植,用于治疗患有多发性骨髓瘤的患者,至少接受过一次治疗,联合使用pomalidomide和地塞米松治疗多发性骨髓瘤患者至少接受过两次治疗,包括来那度胺和蛋白酶体抑制剂单药治疗,用于治疗多发性骨髓瘤患者,这些患者至少接受过三种治疗方法,包括蛋白酶抑制剂(PI)和免疫调节剂,或者是双重耐药剂PI和免疫调节剂。
剂量和给药
用皮质类固醇,退热药和抗组胺药预先给药。稀释并作为静脉输注给药。推荐剂量为16mg/kg实际体重。查看组合和时间表中使用的药物的完整处方信息。管理输液后的药物。
剂量形式和强度
注射:在单剂量小瓶中的100mg/5mL溶液。在单剂量小瓶中加入400mg/20mL溶液。
禁忌症
对daratumumab或制剂的任何组分有严重超敏反应史的患者。
警告和注意事项
输液反应:中断DARZALEX输液用于输液反应的严重程度。在过敏反应或危及生命的输液反应的情况下,永久停止输液,并提供适当的紧急护理。交叉匹配和红细胞抗体筛查的干扰:在开始治疗之前对患者进行分类和筛选。通知血库,患者已收到DARZALEX。中性粒细胞减少症:在治疗期间定期监测完整的血细胞计数。监测中性粒细胞减少症患者的感染迹象。可能需要Dosedelay来恢复中性粒细胞。血小板减少症:在治疗期间定期监测全血细胞计数。可能需要剂量延迟以允许血小板的恢复。
不良反应
最常报告的不良反应(发生率≥20%)为:输液反应,中性粒细胞减少,血小板减少,疲劳,恶心,腹泻,便秘,呕吐,肌肉痉挛,关节痛,背痛,发热,畏寒,头晕,失眠,咳嗽,呼吸困难,外周水肿,外周感觉神经病和上呼吸道感染。
包装提供/存储和处理提供
DARZALEX是一种无色至淡黄色,不含防腐剂的静脉输注溶液,提供:NDC 57894-502-05含有一个100mg/5mL单剂量小瓶NDC 57894-502-20含有一个400mg/20mL单剂量小瓶储存和稳定性储存在2ºC至8ºC(36ºF至46ºF)的冰箱中。不要冻结或摇晃。 避光。
达雷木单抗注射溶液英文版说明书
in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least one prior therapy.Daratumumab was previously granted accelerated approval in November 2015 as monotherapy for patients with multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent, or who are double refractory to a PI and an immunomodulatory agent.The current approval was based on two randomized, open-label trials in which daratumumab was added to standard therapies. The POLLUX trial (also known as MMY3003), demonstrated substantial improvement in progression-free survival (PFS) when daratumumab was added to lenalidomide and dexamethasone compared with lenalidomide and dexamethasone alone. The estimated median PFS had not been reached in the daratumumab arm and was 18.4 months in the control arm (HR=0.37; 95% CI: 0.27, 0.52; p<0.0001), representing a 63% reduction in the risk of disease progression or death in patients treated with daratumumab.Similar results were observed in the CASTOR trial (also known as MMY3004), which compared the combination of daratumumab, bortezomib, and dexamethasone with bortezomib and dexamethasone. The estimated median PFS was not reached in the daratumumab arm and was 7.2 months in the control arm (HR=0.39; 95% CI: 0.28, 0.53; p<0.0001), representing a 61% reduction in the risk of disease progression or death for patients treated with daratumumab.The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3003 were infusion reactions, diarrhea, nausea, fatigue, pyrexia, upper respiratory tract infection, muscle spasm, cough and dyspnea. The most frequently reported adverse reactions (greater than or equal to 20%) in MMY3004 were infusion reactions, diarrhea, peripheral edema, upper respiratory tract infection, peripheral sensory Neutropenia and thrombocytopenia have been added to the Warnings and Precautions of the DARZALEX label.The recommended dose of daratumumab is 16 mg/kg intravenously (calculated on actual body weight).FDA granted daratumumab breakthrough therapy and orphan drug designation, as well as priority review
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 达雷木单抗注射溶液DARZALEX Injection 400mg/2ml(daratumumab)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 达雷木单抗注射溶液DARZALEX Injection 400mg/2ml(daratumumab)