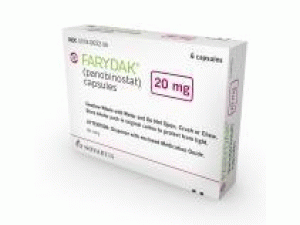
帕比司他胶囊FARYDAK capsules 20mg(panobinostat)
 药店国别:
产地国家:美国
处方药:是
所属类别: 20毫克/胶囊 6胶囊/盒
包装规格: 20毫克/胶囊 6胶囊/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Novartis
原产地英文商品名:Farydak Capsules 20ng/Caps 6Caps/box
原产地英文药品名:panobinostat
中文参考商品译名:Farydak胶囊 20毫克/胶囊 6胶囊/盒
中文参考药品译名:帕比司他
曾用名:LBH589
简介:抗癌新药Farydak(panobinostat,LBH589)首个获FDA批准治疗多发性骨髓瘤的HDAC抑制剂Farydak是第一个HDAC抑制剂被批准治疗多发性骨髓瘤。它意向为患者曾接受至少两个以前标准治疗,包括硼替佐米[bortezomib]和一个免疫调节剂。Farydak是将与硼替佐米,一种类型化疗,和地塞米松,一种抗炎药物联用。FDA的药品评价和研究中心血液学和肿瘤产品室主任Richard Pazdur,M.D.说:“Farydak 有一种新作用机制不同于以前被批准的治疗多发性骨髓瘤药物,使它是一种潜在诱人的为多发性骨髓瘤的治疗备选药,” “Farydak对被批准是特别重要因为它曾减慢多发性骨髓瘤的进展。”Farydak(panobinostat)是一种新型、广谱组蛋白脱乙酰酶(HDAC)抑制剂。通过阻断组蛋白脱乙酰酶(HDAC)发挥作用,该药能够对癌细胞施以严重的应激直至其死亡,而健康细胞则不受影响。批准日期:2015年2月23日 公司:诺华制药FARYDAK®(帕比司他[panobinostat])胶囊,用于口服使用美国初步批准,25%的FARYDAK治疗患者出现严重腹泻。监测症状,进行抗腹泻治疗,中断FARYDAK,然后减少剂量或停止FARYDAK。FARYDAK患者发生严重和致命的心脏缺血事件,严重心律失常和心电图变化。 电解质异常可能导致心律失常。 在临床指出的时候,在基线时和周期性地获得心电图和电解质。
作用机制
FARYDAK是以纳摩尔浓度抑制HDAC的酶活性的组蛋白脱乙酰酶(HDAC)抑制剂。 HDACs催化从组蛋白和一些非组蛋白蛋白的赖氨酸残基去除乙酰基。 HDAC活性的抑制导致组蛋白蛋白的乙酰化增加,导致染色质松弛的表观遗传改变,导致转录激活。在体外,panobinostat引起乙酰化组蛋白和其他蛋白质的积累,诱导一些转化细胞的细胞周期阻滞和/或细胞凋亡。在用panobinostat治疗的小鼠的异种移植物中观察到乙酰化组蛋白水平的增加。与正常细胞相比,Panobinostat对肿瘤细胞的细胞毒性更高。适用范围及用途FARYDAK,组蛋白脱乙酰酶抑制剂与硼替佐米和地塞米松组合用于治疗已接受至少2种以前方案的多发性骨髓瘤患者,包括硼替佐米和免疫调节剂。该指征基于无进展生存加速批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。
剂量和管理
每个21天循环的第1周和第2周每周3次(第1,3,5,8,10和12天),每隔一天口服一次20mg,持续8个周期考虑对具有临床益处的患者进行额外8个周期的持续治疗,除非它们具有未解决的严重或医学上显着的毒性剂量形式和强度胶囊:10mg,15mg和20mg
禁忌症
没有
警告和注意事项
出血:致命和严重的胃肠道和肺部出血病例。根据需要监测血小板计数和输血。
肝毒性:在FARYDAK治疗期间观察到肝功能异常检查时,监测肝酶并调整剂量。
胚胎 - 胎儿毒性:可引起胎儿伤害。建议妇女对胎儿的潜在危害,并采取FARYDAK避免怀孕。
不良反应
临床研究中最常见的不良反应(至少20%的发生率)是腹泻,疲劳,恶心,外周水肿,食欲降低,发热和呕吐。最常见的非血液学实验室异常(发生率≥40%)是低磷血症,低钾血症,低钠血症和增加的肌酐。最常见的血液学实验室异常(发生率≥60%)是血小板减少症,淋巴细胞减少症,白细胞减少症,嗜中性白细胞减少症和贫血。强烈的CYP3A4诱导剂:避免与FARYDAK伴随使用。敏感CYP2D6底物:避免伴随使用FARYDAK。抗心律失常药/ QT延长药物:避免伴随使用。在特定人口中使用肝功能损害:肝功能损害可以增加Panobinostat暴露。减轻轻度或中度肝功能损害患者的FARYDAK剂量。避免用于严重肝损伤患者。
帕比司他胶囊英文版说明书
FARYDAK capsules are packaged in PVC/PCTFE blister packs.10mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0650-0615mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0651-0620mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0652-06FARYDAK, a histone deacetylase inhibitor, in combination with bortezomib and dexamethasone, is indicated for the treatment of patients with multiple myeloma who have received at least 2 prior regimens, including bortezomib and an immunomodulatory agent. This indication is approved under accelerated approval based on progression free survival [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.Farydak DescriptionFARYDAK (panobinostat lactate) is a histone deacetylase inhibitor.The chemical name of panobinostat lactate is 2-Hydroxypropanoic acid, compd. with 2-(E)-N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (1:1).The structural formula is:Panobinostat lactate anhydrous is a white to slightly yellowish or brownish powder. The molecular formula is C21H23N3O2?C3H6O3 (lactate); its molecular weight is 439.51 (as a lactate), equivalent to 349.43 (free base). Panobinostat lactate anhydrous is light sensitive. Panobinostat lactate anhydrous is both chemically and thermodynamically a stable crystalline form with no polymorphic behavior. Panobinostat free base is not chiral and shows no specific optical rotation. Panobinostat lactate anhydrous is slightly soluble in water. Solubility of panobinostat lactate anhydrous is pH-dependent, with the highest solubility in buffer pH 3.0 (citrate).FARYDAK capsules contain 10 mg, 15 mg, or 20 mg panobinostat free base. The inactive ingredients are magnesium stearate, mannitol, microcrystalline cellulose and pregelatinized starch. The capsules contain gelatin, FD&C Blue 1 (10 mg capsules), yellow iron oxide (10 mg and 15 mg capsules), red iron oxide (15 mg and 20 mg capsules) and titanium dioxide.FARYDAK RxGeneric Name and Formulations:Panobinostat 10mg, 15mg, 20mg; caps.Company:Novartis Pharmaceuticals CorpIndications for FARYDAK:Multiple myeloma, in patients who have received at least two prior therapies (including bortezomib and an immunomodulatory agent), in combination with bortezomib and dexamethasone.Adult:Swallow whole with water. Take at same time on scheduled days. Initially 20mg once every other day for 3 doses/wk in Weeks 1 and 2 of each 21-day cycle for up to 8 cycles. Consider 8 more cycles for patients with clinical benefit if no severe or significant toxicity; max 16 cycles (48 wks). Give with bortezomib inj and oral dexamethasone per scheduled day. Hepatic impairment: mild: initially 15mg; moderate: initially 10mg; severe: avoid. Concomitant strong CYP3A inhibitors: initially 10mg. Dose adjustments and modifications for toxicity: see full labeling.Children:Not established.Warnings/Precautions:Risk of severe diarrhea and cardiac toxicities. Monitor hydration and electrolytes at baseline, weekly during therapy, or more as indicated. Initiate antidiarrheals at onset of diarrhea; interrupt dose if 4–6 stools/day. Do not initiate if history of recent MI or unstable angina, QTcF >450msec, significant baseline ST-segment or T-wave abnormalities, active infections. Perform ECG prior to initiation and repeat during treatment as indicated. Correct electrolyte abnormalities prior to initiation and monitor; interrupt if QTcF ≥480msec; discontinue if QT prolongation does not resolve. Serious hemorrhage. Obtain CBC prior to initiation; monitor weekly during therapy or more as indicated. Monitor for infections; treat and consider interruption or discontinuation if diagnosed. Monitor liver function prior to and during treatment; consider dose adjustments if abnormal tests observed. ESRD or dialysis: not studied. Elderly: monitor for toxicity more frequently (esp. GI, myelosuppression, cardiac). Embryo-fetal toxicity. Pregnancy: avoid. Obtain pregnancy test prior to and during treatment. Use effective contraception during and for ≥3 months after last dose; males: use condoms during and for ≥6 months after last dose. Nursing mothers: not recommended.Interactions:Potentiated by strong CYP3A inhibitors (eg, boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, others); see Adults. Avoid star fruit, pomegranate or grapefruit juice. Avoid concomitant strong CYP3A inducers. Avoid concomitant sensitive CYP2D6 substrates (eg, atomoxetine, desipramine, dextromethorphan, metoprolol, nebivolol, perphenazine, tolterodine, venlafaxine) or substrates with narrow therapeutic index (eg, thioridazine, pimozide); if unavoidable, monitor frequently. Concomitant antiarrhythmics or QT prolonging drugs: not recommended. Antiemetics that prolong QT interval (eg, dolasetron, ondansetron, tropisetron): monitor ECG frequently.Pharmacological Class:Histone deacetylase inhibitor.Adverse Reactions:Diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, vomiting, electrolyte imbalance, increased creatinine, thrombocytopenia, lymphopenia, leukopenia, neutropenia, anemia.REMS:YESGeneric Availability:NOHow Supplied:Blister packs—6
药店国别:
产地国家:美国
处方药:是
所属类别: 20毫克/胶囊 6胶囊/盒
包装规格: 20毫克/胶囊 6胶囊/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Novartis
原产地英文商品名:Farydak Capsules 20ng/Caps 6Caps/box
原产地英文药品名:panobinostat
中文参考商品译名:Farydak胶囊 20毫克/胶囊 6胶囊/盒
中文参考药品译名:帕比司他
曾用名:LBH589
简介:抗癌新药Farydak(panobinostat,LBH589)首个获FDA批准治疗多发性骨髓瘤的HDAC抑制剂Farydak是第一个HDAC抑制剂被批准治疗多发性骨髓瘤。它意向为患者曾接受至少两个以前标准治疗,包括硼替佐米[bortezomib]和一个免疫调节剂。Farydak是将与硼替佐米,一种类型化疗,和地塞米松,一种抗炎药物联用。FDA的药品评价和研究中心血液学和肿瘤产品室主任Richard Pazdur,M.D.说:“Farydak 有一种新作用机制不同于以前被批准的治疗多发性骨髓瘤药物,使它是一种潜在诱人的为多发性骨髓瘤的治疗备选药,” “Farydak对被批准是特别重要因为它曾减慢多发性骨髓瘤的进展。”Farydak(panobinostat)是一种新型、广谱组蛋白脱乙酰酶(HDAC)抑制剂。通过阻断组蛋白脱乙酰酶(HDAC)发挥作用,该药能够对癌细胞施以严重的应激直至其死亡,而健康细胞则不受影响。批准日期:2015年2月23日 公司:诺华制药FARYDAK®(帕比司他[panobinostat])胶囊,用于口服使用美国初步批准,25%的FARYDAK治疗患者出现严重腹泻。监测症状,进行抗腹泻治疗,中断FARYDAK,然后减少剂量或停止FARYDAK。FARYDAK患者发生严重和致命的心脏缺血事件,严重心律失常和心电图变化。 电解质异常可能导致心律失常。 在临床指出的时候,在基线时和周期性地获得心电图和电解质。
作用机制
FARYDAK是以纳摩尔浓度抑制HDAC的酶活性的组蛋白脱乙酰酶(HDAC)抑制剂。 HDACs催化从组蛋白和一些非组蛋白蛋白的赖氨酸残基去除乙酰基。 HDAC活性的抑制导致组蛋白蛋白的乙酰化增加,导致染色质松弛的表观遗传改变,导致转录激活。在体外,panobinostat引起乙酰化组蛋白和其他蛋白质的积累,诱导一些转化细胞的细胞周期阻滞和/或细胞凋亡。在用panobinostat治疗的小鼠的异种移植物中观察到乙酰化组蛋白水平的增加。与正常细胞相比,Panobinostat对肿瘤细胞的细胞毒性更高。适用范围及用途FARYDAK,组蛋白脱乙酰酶抑制剂与硼替佐米和地塞米松组合用于治疗已接受至少2种以前方案的多发性骨髓瘤患者,包括硼替佐米和免疫调节剂。该指征基于无进展生存加速批准。继续批准该指征可能取决于确认试验中临床获益的验证和描述。
剂量和管理
每个21天循环的第1周和第2周每周3次(第1,3,5,8,10和12天),每隔一天口服一次20mg,持续8个周期考虑对具有临床益处的患者进行额外8个周期的持续治疗,除非它们具有未解决的严重或医学上显着的毒性剂量形式和强度胶囊:10mg,15mg和20mg
禁忌症
没有
警告和注意事项
出血:致命和严重的胃肠道和肺部出血病例。根据需要监测血小板计数和输血。
肝毒性:在FARYDAK治疗期间观察到肝功能异常检查时,监测肝酶并调整剂量。
胚胎 - 胎儿毒性:可引起胎儿伤害。建议妇女对胎儿的潜在危害,并采取FARYDAK避免怀孕。
不良反应
临床研究中最常见的不良反应(至少20%的发生率)是腹泻,疲劳,恶心,外周水肿,食欲降低,发热和呕吐。最常见的非血液学实验室异常(发生率≥40%)是低磷血症,低钾血症,低钠血症和增加的肌酐。最常见的血液学实验室异常(发生率≥60%)是血小板减少症,淋巴细胞减少症,白细胞减少症,嗜中性白细胞减少症和贫血。强烈的CYP3A4诱导剂:避免与FARYDAK伴随使用。敏感CYP2D6底物:避免伴随使用FARYDAK。抗心律失常药/ QT延长药物:避免伴随使用。在特定人口中使用肝功能损害:肝功能损害可以增加Panobinostat暴露。减轻轻度或中度肝功能损害患者的FARYDAK剂量。避免用于严重肝损伤患者。
帕比司他胶囊英文版说明书
FARYDAK capsules are packaged in PVC/PCTFE blister packs.10mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0650-0615mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0651-0620mg Blister packs containing 6 capsules ………………………….…….NDC 0078-0652-06FARYDAK, a histone deacetylase inhibitor, in combination with bortezomib and dexamethasone, is indicated for the treatment of patients with multiple myeloma who have received at least 2 prior regimens, including bortezomib and an immunomodulatory agent. This indication is approved under accelerated approval based on progression free survival [see Clinical Studies (14.1)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.Farydak DescriptionFARYDAK (panobinostat lactate) is a histone deacetylase inhibitor.The chemical name of panobinostat lactate is 2-Hydroxypropanoic acid, compd. with 2-(E)-N-hydroxy-3-[4-[[[2-(2-methyl-1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (1:1).The structural formula is:Panobinostat lactate anhydrous is a white to slightly yellowish or brownish powder. The molecular formula is C21H23N3O2?C3H6O3 (lactate); its molecular weight is 439.51 (as a lactate), equivalent to 349.43 (free base). Panobinostat lactate anhydrous is light sensitive. Panobinostat lactate anhydrous is both chemically and thermodynamically a stable crystalline form with no polymorphic behavior. Panobinostat free base is not chiral and shows no specific optical rotation. Panobinostat lactate anhydrous is slightly soluble in water. Solubility of panobinostat lactate anhydrous is pH-dependent, with the highest solubility in buffer pH 3.0 (citrate).FARYDAK capsules contain 10 mg, 15 mg, or 20 mg panobinostat free base. The inactive ingredients are magnesium stearate, mannitol, microcrystalline cellulose and pregelatinized starch. The capsules contain gelatin, FD&C Blue 1 (10 mg capsules), yellow iron oxide (10 mg and 15 mg capsules), red iron oxide (15 mg and 20 mg capsules) and titanium dioxide.FARYDAK RxGeneric Name and Formulations:Panobinostat 10mg, 15mg, 20mg; caps.Company:Novartis Pharmaceuticals CorpIndications for FARYDAK:Multiple myeloma, in patients who have received at least two prior therapies (including bortezomib and an immunomodulatory agent), in combination with bortezomib and dexamethasone.Adult:Swallow whole with water. Take at same time on scheduled days. Initially 20mg once every other day for 3 doses/wk in Weeks 1 and 2 of each 21-day cycle for up to 8 cycles. Consider 8 more cycles for patients with clinical benefit if no severe or significant toxicity; max 16 cycles (48 wks). Give with bortezomib inj and oral dexamethasone per scheduled day. Hepatic impairment: mild: initially 15mg; moderate: initially 10mg; severe: avoid. Concomitant strong CYP3A inhibitors: initially 10mg. Dose adjustments and modifications for toxicity: see full labeling.Children:Not established.Warnings/Precautions:Risk of severe diarrhea and cardiac toxicities. Monitor hydration and electrolytes at baseline, weekly during therapy, or more as indicated. Initiate antidiarrheals at onset of diarrhea; interrupt dose if 4–6 stools/day. Do not initiate if history of recent MI or unstable angina, QTcF >450msec, significant baseline ST-segment or T-wave abnormalities, active infections. Perform ECG prior to initiation and repeat during treatment as indicated. Correct electrolyte abnormalities prior to initiation and monitor; interrupt if QTcF ≥480msec; discontinue if QT prolongation does not resolve. Serious hemorrhage. Obtain CBC prior to initiation; monitor weekly during therapy or more as indicated. Monitor for infections; treat and consider interruption or discontinuation if diagnosed. Monitor liver function prior to and during treatment; consider dose adjustments if abnormal tests observed. ESRD or dialysis: not studied. Elderly: monitor for toxicity more frequently (esp. GI, myelosuppression, cardiac). Embryo-fetal toxicity. Pregnancy: avoid. Obtain pregnancy test prior to and during treatment. Use effective contraception during and for ≥3 months after last dose; males: use condoms during and for ≥6 months after last dose. Nursing mothers: not recommended.Interactions:Potentiated by strong CYP3A inhibitors (eg, boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, others); see Adults. Avoid star fruit, pomegranate or grapefruit juice. Avoid concomitant strong CYP3A inducers. Avoid concomitant sensitive CYP2D6 substrates (eg, atomoxetine, desipramine, dextromethorphan, metoprolol, nebivolol, perphenazine, tolterodine, venlafaxine) or substrates with narrow therapeutic index (eg, thioridazine, pimozide); if unavoidable, monitor frequently. Concomitant antiarrhythmics or QT prolonging drugs: not recommended. Antiemetics that prolong QT interval (eg, dolasetron, ondansetron, tropisetron): monitor ECG frequently.Pharmacological Class:Histone deacetylase inhibitor.Adverse Reactions:Diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, vomiting, electrolyte imbalance, increased creatinine, thrombocytopenia, lymphopenia, leukopenia, neutropenia, anemia.REMS:YESGeneric Availability:NOHow Supplied:Blister packs—6
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 帕比司他胶囊FARYDAK capsules 20mg(panobinostat)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 帕比司他胶囊FARYDAK capsules 20mg(panobinostat)