卡非佐米KYPROLIS 40mg Vial(Carfilzomib)
产地国家:日本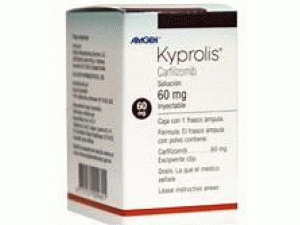 处方药:是
所属类别: 40毫克/瓶
包装规格: 40毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Ono Pharmaceutical Co.Ltd.
原产地英文商品名:Kyprolis(カイプロリス点滴静注用)40mg/Vial
原产地英文药品名:Carfilzomib
中文参考商品译名:Kyprolis(カイプロリス点滴静注用)40毫克/瓶
中文参考药品译名:卡非佐米
曾用名:
简介:部份中文KYPROLIS 处方资料(仅供参考)药物分类名称─抗肿瘤剂──蛋白酶体抑制剂批准日期:2016年8月商標名KYPROLIS一般名カルフィルゾミブ(Carfilzomib)化学名N-{(2S)-2-[(Morpholin-4-ylacetyl)amino]-4-phenyl-butanoyl}-L-leucyl-L-phenylalanin-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}amide構造式分子式C40H57N5O7分子量719.91性状本产品为白色至灰白色固体,易溶于N-甲基-2-吡咯烷酮,N,N-二甲基甲酰胺或二甲基亚砜,微溶于甲醇或乙醇(99.5),微溶于乙腈或2-丙醇 它很难融化,几乎不溶于水。审批条件制定药品风险管理计划并适当执行。2.由于在日本的临床试验数量非常有限,所以通过对所有病例进行使用情况调查,直到一定数量病例的数据在制造和销售之后积累, 除了掌握使用这种药物的患者的背景资料之外,还应尽快收集这种药物的安全性和有效性数据,并采取必要的措施正确使用这种药物。
药效药理
1. 作用机序Califilzomib通过抑制蛋白酶体的胰凝乳蛋白酶样活性来诱导肿瘤细胞凋亡并抑制肿瘤生长。
2. 抗肿瘤效果
(1) Calfirzomib在体外试验中抑制来源于人多发性骨髓瘤的MM.1S和RPMI-8226细胞系的增殖。 此外,它抑制对MM.1S和对地塞米松具有抗性的美法仑的耐药性的RPMI-8226细胞系的增殖。
(2) Calfirzomib在用MM.1S细胞系皮下移植的免疫受损小鼠中抑制肿瘤生长。适应病症复发性或难治性多发性骨髓瘤
用法与用量
在使用来那度胺和地塞米松组合的情况下:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。 28天为是一个周期,并且管理重复了12个周期。13个周期后,在第1,2,15和16天每天静脉滴注一次,药物撤回12天。第一周期的第一天和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一周期的体重是27mg/m 2(体表面积),并且在10分钟内静脉滴注。 顺便提一下,根据患者的状况来减轻体重。当联合使用地塞米松时:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。用这28天重复给药1个周期。第一次循环的第一和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一次循环后56mg/m 2(体表面积),并且在30分钟内静脉滴注。 另外,根据患者的状况来减轻体重。
包装规格
静脉滴注10毫克:1瓶40毫克:1瓶
卡非佐米英文版说明书
Manufacturing and marketing approval for recurrent or refractory multiple myeloma Application for partial change approvalOno Pharmaceutical Co., Ltd. (Headquarters: Chuo-ku, Osaka-shi, President and CEO: Akira Sagara, hereinafter referred to as "our company") will launch in Japan on August 31 as a remedy for recurrent or refractory multiple myeloma We have applied for a partial change approval on domestic marketing approval matters on August 25 regarding the proteasome inhibitor "Kyprolith IV for intravenous infusion 10 mg, 40 mg" (common name: Calfirzomib, hereinafter referred to as Kiprolith).KYPROLIS(carfilzomib)IMPORTANT SAFETY INFORMATION AND APPROVED USES KYPROLIS® (carfilzomib) can cause serious side effects:•Heart problems: KYPROLIS can cause heart problems or worsen pre-existing heart conditions. Death due to cardiac arrest has occurred within one day of KYPROLIS administration. Before starting KYPROLIS, you should have a full medical work-up (including blood pressure and fluid management). You should be closely monitored during treatment.•Kidney problems: There have been reports of sudden kidney failure in patients receiving KYPROLIS. Your kidney function should be closely monitored during treatment.•Tumor lysis syndrome (TLS): Cases of TLS have been reported in patients receiving KYPROLIS, including fatalities. You should be closely monitored during treatment for any signs of TLS.•Lung damage: Cases of lung damage have been reported in patients receiving KYPROLIS, including fatal cases.•Pulmonary hypertension (high blood pressure in the lungs): There have been reports of pulmonary hypertension in patients receiving KYPROLIS.•Lung complications: Shortness of breath was reported in patients receiving KYPROLIS. Your lung function should be closely monitored during treatment.•High blood pressure: Cases of high blood pressure, including fatal cases, have been reported in patients receiving KYPROLIS. Your blood pressure should be closely monitored during treatment.•Blood clots: There have been reports of blood clots in patients receiving KYPROLIS. If you are at high risk for blood clots, your doctor can recommend ways to lower the risk.•If you are using KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone, your doctor should assess and may prescribe another medicine to help lower your risk for blood clots.•If you are using birth control pills or other medical forms of birth control associated with a risk of blood clots, talk to your doctor and consider a different method of birth control during treatment with KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone.•Infusion reactions: Symptoms of infusion reactions included fever, chills, joint pain, muscle pain, facial flushing and/or swelling, vomiting, weakness, shortness of breath, low blood pressure, fainting, chest tightness, and chest pain. These symptoms can occur immediately following infusion or up to 24 hours after administration of KYPROLIS. If you experience any of these symptoms, contact your doctor immediately.•Severe bleeding problems: Fatal or serious cases of bleeding problems have been reported in patients receiving KYPROLIS. Your doctor should monitor your signs and symptoms of blood loss.•Very low platelet count: Low platelet levels can cause unusual bruising and bleeding. You should have regular blood tests to check your platelet count during treatment.•Liver problems: Cases of liver failure, including fatal cases, have been reported in patients receiving KYPROLIS. Your liver function should be closely monitored during treatment.•Blood problems: Cases of a blood disease called thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal cases, have been reported in patients who received KYPROLIS. Your doctor should monitor your signs and symptoms.•Brain problems: A nerve disease called Posterior Reversible Encephalopathy Syndrome (PRES), formerly called Reversible Posterior Leukoencephalopathy Syndrome (RPLS), has been reported in patients receiving KYPROLIS. It can cause seizure, headache, lack of energy, confusion, blindness, altered consciousness, and other visual and nerve disturbances, along with high blood pressure. Your doctor should monitor your signs and symptoms.•KYPROLIS should not be combined with melphalan and prednisone: Newly diagnosed transplant ineligible multiple myeloma patients have shown an increased risk of serious and fatal side effects when using KYPROLIS in combination with melphalan and prednisone.•Possible fetal harm: KYPROLIS can cause harm to a fetus (unborn baby) when given to a pregnant woman. Women should avoid becoming pregnant during treatment with KYPROLIS. Men should avoid fathering a child during treatment with KYPROLIS. KYPROLIS can cause harm to a fetus if used during pregnancy or if you or your partner become pregnant during treatment with KYPROLIS.You should contact your doctor immediately if you experience any of the following:•Shortness of breath•Prolonged, unusual or excessive bleeding•Yellowing of the skin and/or eyes (jaundice)•Headaches, confusion, seizures, or loss of sight•Pregnancy (women should not receive KYPROLIS if they are pregnant or breastfeeding)•Any other side effect that bothers you or does not go awayWhat are the possible side effects of KYPROLIS?•The most common side effects occurring in at least 20% of patients receiving KYPROLIS in the combination therapy trials are: low red blood cell count, low white blood cell count, diarrhea, difficulty breathing, tiredness (fatigue), low platelets, fever, sleeplessness (insomnia), muscle spasm, cough, upper airway (respiratory tract) infection, and decreased potassium levels.•The most common side effects occurring in at least 20% of patients receiving KYPROLIS when used alone (monotherapy) in trials are: low red blood cell count, tiredness (fatigue), low platelets, nausea, fever, difficulty breathing, diarrhea, headache, cough, swelling of the lower legs or hands.These are not all the possible side effects of KYPROLIS. For more information, ask your doctor or pharmacist.Please talk to your doctor and see the full Product Information for additional information.APPROVED USES•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one to three previous treatments for multiple myeloma. KYPROLIS is approved for use in combination with dexamethasone or with lenalidomide plus dexamethasone, which are other medicines used to treat multiple myeloma.•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one or more previous treatments for multiple myeloma. KYPROLIS is approved for use alone to treat relapsed or refractory multiple myeloma.
处方药:是
所属类别: 40毫克/瓶
包装规格: 40毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Ono Pharmaceutical Co.Ltd.
原产地英文商品名:Kyprolis(カイプロリス点滴静注用)40mg/Vial
原产地英文药品名:Carfilzomib
中文参考商品译名:Kyprolis(カイプロリス点滴静注用)40毫克/瓶
中文参考药品译名:卡非佐米
曾用名:
简介:部份中文KYPROLIS 处方资料(仅供参考)药物分类名称─抗肿瘤剂──蛋白酶体抑制剂批准日期:2016年8月商標名KYPROLIS一般名カルフィルゾミブ(Carfilzomib)化学名N-{(2S)-2-[(Morpholin-4-ylacetyl)amino]-4-phenyl-butanoyl}-L-leucyl-L-phenylalanin-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}amide構造式分子式C40H57N5O7分子量719.91性状本产品为白色至灰白色固体,易溶于N-甲基-2-吡咯烷酮,N,N-二甲基甲酰胺或二甲基亚砜,微溶于甲醇或乙醇(99.5),微溶于乙腈或2-丙醇 它很难融化,几乎不溶于水。审批条件制定药品风险管理计划并适当执行。2.由于在日本的临床试验数量非常有限,所以通过对所有病例进行使用情况调查,直到一定数量病例的数据在制造和销售之后积累, 除了掌握使用这种药物的患者的背景资料之外,还应尽快收集这种药物的安全性和有效性数据,并采取必要的措施正确使用这种药物。
药效药理
1. 作用机序Califilzomib通过抑制蛋白酶体的胰凝乳蛋白酶样活性来诱导肿瘤细胞凋亡并抑制肿瘤生长。
2. 抗肿瘤效果
(1) Calfirzomib在体外试验中抑制来源于人多发性骨髓瘤的MM.1S和RPMI-8226细胞系的增殖。 此外,它抑制对MM.1S和对地塞米松具有抗性的美法仑的耐药性的RPMI-8226细胞系的增殖。
(2) Calfirzomib在用MM.1S细胞系皮下移植的免疫受损小鼠中抑制肿瘤生长。适应病症复发性或难治性多发性骨髓瘤
用法与用量
在使用来那度胺和地塞米松组合的情况下:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。 28天为是一个周期,并且管理重复了12个周期。13个周期后,在第1,2,15和16天每天静脉滴注一次,药物撤回12天。第一周期的第一天和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一周期的体重是27mg/m 2(体表面积),并且在10分钟内静脉滴注。 顺便提一下,根据患者的状况来减轻体重。当联合使用地塞米松时:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。用这28天重复给药1个周期。第一次循环的第一和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一次循环后56mg/m 2(体表面积),并且在30分钟内静脉滴注。 另外,根据患者的状况来减轻体重。
包装规格
静脉滴注10毫克:1瓶40毫克:1瓶
卡非佐米英文版说明书
Manufacturing and marketing approval for recurrent or refractory multiple myeloma Application for partial change approvalOno Pharmaceutical Co., Ltd. (Headquarters: Chuo-ku, Osaka-shi, President and CEO: Akira Sagara, hereinafter referred to as "our company") will launch in Japan on August 31 as a remedy for recurrent or refractory multiple myeloma We have applied for a partial change approval on domestic marketing approval matters on August 25 regarding the proteasome inhibitor "Kyprolith IV for intravenous infusion 10 mg, 40 mg" (common name: Calfirzomib, hereinafter referred to as Kiprolith).KYPROLIS(carfilzomib)IMPORTANT SAFETY INFORMATION AND APPROVED USES KYPROLIS® (carfilzomib) can cause serious side effects:•Heart problems: KYPROLIS can cause heart problems or worsen pre-existing heart conditions. Death due to cardiac arrest has occurred within one day of KYPROLIS administration. Before starting KYPROLIS, you should have a full medical work-up (including blood pressure and fluid management). You should be closely monitored during treatment.•Kidney problems: There have been reports of sudden kidney failure in patients receiving KYPROLIS. Your kidney function should be closely monitored during treatment.•Tumor lysis syndrome (TLS): Cases of TLS have been reported in patients receiving KYPROLIS, including fatalities. You should be closely monitored during treatment for any signs of TLS.•Lung damage: Cases of lung damage have been reported in patients receiving KYPROLIS, including fatal cases.•Pulmonary hypertension (high blood pressure in the lungs): There have been reports of pulmonary hypertension in patients receiving KYPROLIS.•Lung complications: Shortness of breath was reported in patients receiving KYPROLIS. Your lung function should be closely monitored during treatment.•High blood pressure: Cases of high blood pressure, including fatal cases, have been reported in patients receiving KYPROLIS. Your blood pressure should be closely monitored during treatment.•Blood clots: There have been reports of blood clots in patients receiving KYPROLIS. If you are at high risk for blood clots, your doctor can recommend ways to lower the risk.•If you are using KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone, your doctor should assess and may prescribe another medicine to help lower your risk for blood clots.•If you are using birth control pills or other medical forms of birth control associated with a risk of blood clots, talk to your doctor and consider a different method of birth control during treatment with KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone.•Infusion reactions: Symptoms of infusion reactions included fever, chills, joint pain, muscle pain, facial flushing and/or swelling, vomiting, weakness, shortness of breath, low blood pressure, fainting, chest tightness, and chest pain. These symptoms can occur immediately following infusion or up to 24 hours after administration of KYPROLIS. If you experience any of these symptoms, contact your doctor immediately.•Severe bleeding problems: Fatal or serious cases of bleeding problems have been reported in patients receiving KYPROLIS. Your doctor should monitor your signs and symptoms of blood loss.•Very low platelet count: Low platelet levels can cause unusual bruising and bleeding. You should have regular blood tests to check your platelet count during treatment.•Liver problems: Cases of liver failure, including fatal cases, have been reported in patients receiving KYPROLIS. Your liver function should be closely monitored during treatment.•Blood problems: Cases of a blood disease called thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal cases, have been reported in patients who received KYPROLIS. Your doctor should monitor your signs and symptoms.•Brain problems: A nerve disease called Posterior Reversible Encephalopathy Syndrome (PRES), formerly called Reversible Posterior Leukoencephalopathy Syndrome (RPLS), has been reported in patients receiving KYPROLIS. It can cause seizure, headache, lack of energy, confusion, blindness, altered consciousness, and other visual and nerve disturbances, along with high blood pressure. Your doctor should monitor your signs and symptoms.•KYPROLIS should not be combined with melphalan and prednisone: Newly diagnosed transplant ineligible multiple myeloma patients have shown an increased risk of serious and fatal side effects when using KYPROLIS in combination with melphalan and prednisone.•Possible fetal harm: KYPROLIS can cause harm to a fetus (unborn baby) when given to a pregnant woman. Women should avoid becoming pregnant during treatment with KYPROLIS. Men should avoid fathering a child during treatment with KYPROLIS. KYPROLIS can cause harm to a fetus if used during pregnancy or if you or your partner become pregnant during treatment with KYPROLIS.You should contact your doctor immediately if you experience any of the following:•Shortness of breath•Prolonged, unusual or excessive bleeding•Yellowing of the skin and/or eyes (jaundice)•Headaches, confusion, seizures, or loss of sight•Pregnancy (women should not receive KYPROLIS if they are pregnant or breastfeeding)•Any other side effect that bothers you or does not go awayWhat are the possible side effects of KYPROLIS?•The most common side effects occurring in at least 20% of patients receiving KYPROLIS in the combination therapy trials are: low red blood cell count, low white blood cell count, diarrhea, difficulty breathing, tiredness (fatigue), low platelets, fever, sleeplessness (insomnia), muscle spasm, cough, upper airway (respiratory tract) infection, and decreased potassium levels.•The most common side effects occurring in at least 20% of patients receiving KYPROLIS when used alone (monotherapy) in trials are: low red blood cell count, tiredness (fatigue), low platelets, nausea, fever, difficulty breathing, diarrhea, headache, cough, swelling of the lower legs or hands.These are not all the possible side effects of KYPROLIS. For more information, ask your doctor or pharmacist.Please talk to your doctor and see the full Product Information for additional information.APPROVED USES•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one to three previous treatments for multiple myeloma. KYPROLIS is approved for use in combination with dexamethasone or with lenalidomide plus dexamethasone, which are other medicines used to treat multiple myeloma.•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one or more previous treatments for multiple myeloma. KYPROLIS is approved for use alone to treat relapsed or refractory multiple myeloma.
 处方药:是
所属类别: 40毫克/瓶
包装规格: 40毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Ono Pharmaceutical Co.Ltd.
原产地英文商品名:Kyprolis(カイプロリス点滴静注用)40mg/Vial
原产地英文药品名:Carfilzomib
中文参考商品译名:Kyprolis(カイプロリス点滴静注用)40毫克/瓶
中文参考药品译名:卡非佐米
曾用名:
简介:部份中文KYPROLIS 处方资料(仅供参考)药物分类名称─抗肿瘤剂──蛋白酶体抑制剂批准日期:2016年8月商標名KYPROLIS一般名カルフィルゾミブ(Carfilzomib)化学名N-{(2S)-2-[(Morpholin-4-ylacetyl)amino]-4-phenyl-butanoyl}-L-leucyl-L-phenylalanin-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}amide構造式分子式C40H57N5O7分子量719.91性状本产品为白色至灰白色固体,易溶于N-甲基-2-吡咯烷酮,N,N-二甲基甲酰胺或二甲基亚砜,微溶于甲醇或乙醇(99.5),微溶于乙腈或2-丙醇 它很难融化,几乎不溶于水。审批条件制定药品风险管理计划并适当执行。2.由于在日本的临床试验数量非常有限,所以通过对所有病例进行使用情况调查,直到一定数量病例的数据在制造和销售之后积累, 除了掌握使用这种药物的患者的背景资料之外,还应尽快收集这种药物的安全性和有效性数据,并采取必要的措施正确使用这种药物。
药效药理
1. 作用机序Califilzomib通过抑制蛋白酶体的胰凝乳蛋白酶样活性来诱导肿瘤细胞凋亡并抑制肿瘤生长。
2. 抗肿瘤效果
(1) Calfirzomib在体外试验中抑制来源于人多发性骨髓瘤的MM.1S和RPMI-8226细胞系的增殖。 此外,它抑制对MM.1S和对地塞米松具有抗性的美法仑的耐药性的RPMI-8226细胞系的增殖。
(2) Calfirzomib在用MM.1S细胞系皮下移植的免疫受损小鼠中抑制肿瘤生长。适应病症复发性或难治性多发性骨髓瘤
用法与用量
在使用来那度胺和地塞米松组合的情况下:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。 28天为是一个周期,并且管理重复了12个周期。13个周期后,在第1,2,15和16天每天静脉滴注一次,药物撤回12天。第一周期的第一天和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一周期的体重是27mg/m 2(体表面积),并且在10分钟内静脉滴注。 顺便提一下,根据患者的状况来减轻体重。当联合使用地塞米松时:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。用这28天重复给药1个周期。第一次循环的第一和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一次循环后56mg/m 2(体表面积),并且在30分钟内静脉滴注。 另外,根据患者的状况来减轻体重。
包装规格
静脉滴注10毫克:1瓶40毫克:1瓶
卡非佐米英文版说明书
Manufacturing and marketing approval for recurrent or refractory multiple myeloma Application for partial change approvalOno Pharmaceutical Co., Ltd. (Headquarters: Chuo-ku, Osaka-shi, President and CEO: Akira Sagara, hereinafter referred to as "our company") will launch in Japan on August 31 as a remedy for recurrent or refractory multiple myeloma We have applied for a partial change approval on domestic marketing approval matters on August 25 regarding the proteasome inhibitor "Kyprolith IV for intravenous infusion 10 mg, 40 mg" (common name: Calfirzomib, hereinafter referred to as Kiprolith).KYPROLIS(carfilzomib)IMPORTANT SAFETY INFORMATION AND APPROVED USES KYPROLIS® (carfilzomib) can cause serious side effects:•Heart problems: KYPROLIS can cause heart problems or worsen pre-existing heart conditions. Death due to cardiac arrest has occurred within one day of KYPROLIS administration. Before starting KYPROLIS, you should have a full medical work-up (including blood pressure and fluid management). You should be closely monitored during treatment.•Kidney problems: There have been reports of sudden kidney failure in patients receiving KYPROLIS. Your kidney function should be closely monitored during treatment.•Tumor lysis syndrome (TLS): Cases of TLS have been reported in patients receiving KYPROLIS, including fatalities. You should be closely monitored during treatment for any signs of TLS.•Lung damage: Cases of lung damage have been reported in patients receiving KYPROLIS, including fatal cases.•Pulmonary hypertension (high blood pressure in the lungs): There have been reports of pulmonary hypertension in patients receiving KYPROLIS.•Lung complications: Shortness of breath was reported in patients receiving KYPROLIS. Your lung function should be closely monitored during treatment.•High blood pressure: Cases of high blood pressure, including fatal cases, have been reported in patients receiving KYPROLIS. Your blood pressure should be closely monitored during treatment.•Blood clots: There have been reports of blood clots in patients receiving KYPROLIS. If you are at high risk for blood clots, your doctor can recommend ways to lower the risk.•If you are using KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone, your doctor should assess and may prescribe another medicine to help lower your risk for blood clots.•If you are using birth control pills or other medical forms of birth control associated with a risk of blood clots, talk to your doctor and consider a different method of birth control during treatment with KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone.•Infusion reactions: Symptoms of infusion reactions included fever, chills, joint pain, muscle pain, facial flushing and/or swelling, vomiting, weakness, shortness of breath, low blood pressure, fainting, chest tightness, and chest pain. These symptoms can occur immediately following infusion or up to 24 hours after administration of KYPROLIS. If you experience any of these symptoms, contact your doctor immediately.•Severe bleeding problems: Fatal or serious cases of bleeding problems have been reported in patients receiving KYPROLIS. Your doctor should monitor your signs and symptoms of blood loss.•Very low platelet count: Low platelet levels can cause unusual bruising and bleeding. You should have regular blood tests to check your platelet count during treatment.•Liver problems: Cases of liver failure, including fatal cases, have been reported in patients receiving KYPROLIS. Your liver function should be closely monitored during treatment.•Blood problems: Cases of a blood disease called thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal cases, have been reported in patients who received KYPROLIS. Your doctor should monitor your signs and symptoms.•Brain problems: A nerve disease called Posterior Reversible Encephalopathy Syndrome (PRES), formerly called Reversible Posterior Leukoencephalopathy Syndrome (RPLS), has been reported in patients receiving KYPROLIS. It can cause seizure, headache, lack of energy, confusion, blindness, altered consciousness, and other visual and nerve disturbances, along with high blood pressure. Your doctor should monitor your signs and symptoms.•KYPROLIS should not be combined with melphalan and prednisone: Newly diagnosed transplant ineligible multiple myeloma patients have shown an increased risk of serious and fatal side effects when using KYPROLIS in combination with melphalan and prednisone.•Possible fetal harm: KYPROLIS can cause harm to a fetus (unborn baby) when given to a pregnant woman. Women should avoid becoming pregnant during treatment with KYPROLIS. Men should avoid fathering a child during treatment with KYPROLIS. KYPROLIS can cause harm to a fetus if used during pregnancy or if you or your partner become pregnant during treatment with KYPROLIS.You should contact your doctor immediately if you experience any of the following:•Shortness of breath•Prolonged, unusual or excessive bleeding•Yellowing of the skin and/or eyes (jaundice)•Headaches, confusion, seizures, or loss of sight•Pregnancy (women should not receive KYPROLIS if they are pregnant or breastfeeding)•Any other side effect that bothers you or does not go awayWhat are the possible side effects of KYPROLIS?•The most common side effects occurring in at least 20% of patients receiving KYPROLIS in the combination therapy trials are: low red blood cell count, low white blood cell count, diarrhea, difficulty breathing, tiredness (fatigue), low platelets, fever, sleeplessness (insomnia), muscle spasm, cough, upper airway (respiratory tract) infection, and decreased potassium levels.•The most common side effects occurring in at least 20% of patients receiving KYPROLIS when used alone (monotherapy) in trials are: low red blood cell count, tiredness (fatigue), low platelets, nausea, fever, difficulty breathing, diarrhea, headache, cough, swelling of the lower legs or hands.These are not all the possible side effects of KYPROLIS. For more information, ask your doctor or pharmacist.Please talk to your doctor and see the full Product Information for additional information.APPROVED USES•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one to three previous treatments for multiple myeloma. KYPROLIS is approved for use in combination with dexamethasone or with lenalidomide plus dexamethasone, which are other medicines used to treat multiple myeloma.•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one or more previous treatments for multiple myeloma. KYPROLIS is approved for use alone to treat relapsed or refractory multiple myeloma.
处方药:是
所属类别: 40毫克/瓶
包装规格: 40毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Ono Pharmaceutical Co.Ltd.
原产地英文商品名:Kyprolis(カイプロリス点滴静注用)40mg/Vial
原产地英文药品名:Carfilzomib
中文参考商品译名:Kyprolis(カイプロリス点滴静注用)40毫克/瓶
中文参考药品译名:卡非佐米
曾用名:
简介:部份中文KYPROLIS 处方资料(仅供参考)药物分类名称─抗肿瘤剂──蛋白酶体抑制剂批准日期:2016年8月商標名KYPROLIS一般名カルフィルゾミブ(Carfilzomib)化学名N-{(2S)-2-[(Morpholin-4-ylacetyl)amino]-4-phenyl-butanoyl}-L-leucyl-L-phenylalanin-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}amide構造式分子式C40H57N5O7分子量719.91性状本产品为白色至灰白色固体,易溶于N-甲基-2-吡咯烷酮,N,N-二甲基甲酰胺或二甲基亚砜,微溶于甲醇或乙醇(99.5),微溶于乙腈或2-丙醇 它很难融化,几乎不溶于水。审批条件制定药品风险管理计划并适当执行。2.由于在日本的临床试验数量非常有限,所以通过对所有病例进行使用情况调查,直到一定数量病例的数据在制造和销售之后积累, 除了掌握使用这种药物的患者的背景资料之外,还应尽快收集这种药物的安全性和有效性数据,并采取必要的措施正确使用这种药物。
药效药理
1. 作用机序Califilzomib通过抑制蛋白酶体的胰凝乳蛋白酶样活性来诱导肿瘤细胞凋亡并抑制肿瘤生长。
2. 抗肿瘤效果
(1) Calfirzomib在体外试验中抑制来源于人多发性骨髓瘤的MM.1S和RPMI-8226细胞系的增殖。 此外,它抑制对MM.1S和对地塞米松具有抗性的美法仑的耐药性的RPMI-8226细胞系的增殖。
(2) Calfirzomib在用MM.1S细胞系皮下移植的免疫受损小鼠中抑制肿瘤生长。适应病症复发性或难治性多发性骨髓瘤
用法与用量
在使用来那度胺和地塞米松组合的情况下:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。 28天为是一个周期,并且管理重复了12个周期。13个周期后,在第1,2,15和16天每天静脉滴注一次,药物撤回12天。第一周期的第一天和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一周期的体重是27mg/m 2(体表面积),并且在10分钟内静脉滴注。 顺便提一下,根据患者的状况来减轻体重。当联合使用地塞米松时:成人;在第1,2,8,9,15和16天每天一次接受静脉滴注,并且撤回12天。用这28天重复给药1个周期。第一次循环的第一和第二天,该药物的剂量应该是20mg/m 2(体表面积),第一次循环后56mg/m 2(体表面积),并且在30分钟内静脉滴注。 另外,根据患者的状况来减轻体重。
包装规格
静脉滴注10毫克:1瓶40毫克:1瓶
卡非佐米英文版说明书
Manufacturing and marketing approval for recurrent or refractory multiple myeloma Application for partial change approvalOno Pharmaceutical Co., Ltd. (Headquarters: Chuo-ku, Osaka-shi, President and CEO: Akira Sagara, hereinafter referred to as "our company") will launch in Japan on August 31 as a remedy for recurrent or refractory multiple myeloma We have applied for a partial change approval on domestic marketing approval matters on August 25 regarding the proteasome inhibitor "Kyprolith IV for intravenous infusion 10 mg, 40 mg" (common name: Calfirzomib, hereinafter referred to as Kiprolith).KYPROLIS(carfilzomib)IMPORTANT SAFETY INFORMATION AND APPROVED USES KYPROLIS® (carfilzomib) can cause serious side effects:•Heart problems: KYPROLIS can cause heart problems or worsen pre-existing heart conditions. Death due to cardiac arrest has occurred within one day of KYPROLIS administration. Before starting KYPROLIS, you should have a full medical work-up (including blood pressure and fluid management). You should be closely monitored during treatment.•Kidney problems: There have been reports of sudden kidney failure in patients receiving KYPROLIS. Your kidney function should be closely monitored during treatment.•Tumor lysis syndrome (TLS): Cases of TLS have been reported in patients receiving KYPROLIS, including fatalities. You should be closely monitored during treatment for any signs of TLS.•Lung damage: Cases of lung damage have been reported in patients receiving KYPROLIS, including fatal cases.•Pulmonary hypertension (high blood pressure in the lungs): There have been reports of pulmonary hypertension in patients receiving KYPROLIS.•Lung complications: Shortness of breath was reported in patients receiving KYPROLIS. Your lung function should be closely monitored during treatment.•High blood pressure: Cases of high blood pressure, including fatal cases, have been reported in patients receiving KYPROLIS. Your blood pressure should be closely monitored during treatment.•Blood clots: There have been reports of blood clots in patients receiving KYPROLIS. If you are at high risk for blood clots, your doctor can recommend ways to lower the risk.•If you are using KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone, your doctor should assess and may prescribe another medicine to help lower your risk for blood clots.•If you are using birth control pills or other medical forms of birth control associated with a risk of blood clots, talk to your doctor and consider a different method of birth control during treatment with KYPROLIS in combination with dexamethasone or with lenalidomide plus dexamethasone.•Infusion reactions: Symptoms of infusion reactions included fever, chills, joint pain, muscle pain, facial flushing and/or swelling, vomiting, weakness, shortness of breath, low blood pressure, fainting, chest tightness, and chest pain. These symptoms can occur immediately following infusion or up to 24 hours after administration of KYPROLIS. If you experience any of these symptoms, contact your doctor immediately.•Severe bleeding problems: Fatal or serious cases of bleeding problems have been reported in patients receiving KYPROLIS. Your doctor should monitor your signs and symptoms of blood loss.•Very low platelet count: Low platelet levels can cause unusual bruising and bleeding. You should have regular blood tests to check your platelet count during treatment.•Liver problems: Cases of liver failure, including fatal cases, have been reported in patients receiving KYPROLIS. Your liver function should be closely monitored during treatment.•Blood problems: Cases of a blood disease called thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal cases, have been reported in patients who received KYPROLIS. Your doctor should monitor your signs and symptoms.•Brain problems: A nerve disease called Posterior Reversible Encephalopathy Syndrome (PRES), formerly called Reversible Posterior Leukoencephalopathy Syndrome (RPLS), has been reported in patients receiving KYPROLIS. It can cause seizure, headache, lack of energy, confusion, blindness, altered consciousness, and other visual and nerve disturbances, along with high blood pressure. Your doctor should monitor your signs and symptoms.•KYPROLIS should not be combined with melphalan and prednisone: Newly diagnosed transplant ineligible multiple myeloma patients have shown an increased risk of serious and fatal side effects when using KYPROLIS in combination with melphalan and prednisone.•Possible fetal harm: KYPROLIS can cause harm to a fetus (unborn baby) when given to a pregnant woman. Women should avoid becoming pregnant during treatment with KYPROLIS. Men should avoid fathering a child during treatment with KYPROLIS. KYPROLIS can cause harm to a fetus if used during pregnancy or if you or your partner become pregnant during treatment with KYPROLIS.You should contact your doctor immediately if you experience any of the following:•Shortness of breath•Prolonged, unusual or excessive bleeding•Yellowing of the skin and/or eyes (jaundice)•Headaches, confusion, seizures, or loss of sight•Pregnancy (women should not receive KYPROLIS if they are pregnant or breastfeeding)•Any other side effect that bothers you or does not go awayWhat are the possible side effects of KYPROLIS?•The most common side effects occurring in at least 20% of patients receiving KYPROLIS in the combination therapy trials are: low red blood cell count, low white blood cell count, diarrhea, difficulty breathing, tiredness (fatigue), low platelets, fever, sleeplessness (insomnia), muscle spasm, cough, upper airway (respiratory tract) infection, and decreased potassium levels.•The most common side effects occurring in at least 20% of patients receiving KYPROLIS when used alone (monotherapy) in trials are: low red blood cell count, tiredness (fatigue), low platelets, nausea, fever, difficulty breathing, diarrhea, headache, cough, swelling of the lower legs or hands.These are not all the possible side effects of KYPROLIS. For more information, ask your doctor or pharmacist.Please talk to your doctor and see the full Product Information for additional information.APPROVED USES•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one to three previous treatments for multiple myeloma. KYPROLIS is approved for use in combination with dexamethasone or with lenalidomide plus dexamethasone, which are other medicines used to treat multiple myeloma.•KYPROLIS® (carfilzomib) is a prescription medication used to treat patients with relapsed or refractory multiple myeloma who have received one or more previous treatments for multiple myeloma. KYPROLIS is approved for use alone to treat relapsed or refractory multiple myeloma.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡非佐米KYPROLIS 40mg Vial(Carfilzomib)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡非佐米KYPROLIS 40mg Vial(Carfilzomib)