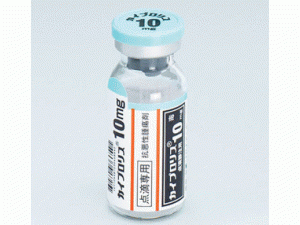
卡非佐米冻干粉注射剂Kyprolis Injection 10mg(carfilzomib)
 药店国别:
产地国家:美国
处方药:是
所属类别: 10毫克/瓶 1瓶
包装规格: 10毫克/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:AMGEN USA INC
原产地英文商品名:KYPROLIS 10MG SDV LYO PWD 1/EA
原产地英文药品名:CARFILZOMIB
中文参考商品译名:KYPROLIS冻干粉注射剂 10毫克/瓶 1瓶
中文参考药品译名:卡非佐米
曾用名:
简介:美国FDA批准蛋白酶抑制剂carfilzomib(Kyprolis)用于接受过其他疗法但效果不佳的多发性骨髓瘤患者的治疗。批准该药用于至少两种已有疗法 (其中之一必须为硼替佐米,Velcade) 无效的复发性或难治性骨髓瘤患者的治疗。FDA的药物评价和研究中心血液学和肿瘤室主任Richard Pazdur,M.D 说:“Kyprolis的批准对尽管使用可得到的治疗其疾病已进展的多发性骨髓瘤患者提供治疗选择,”“在过去十年通过为多发性骨髓瘤药物不断的进展,提供这种疾病改善治疗。我们受到鼓舞。”批准日期2012年7月20日;公司:Onyx Pharmaceuticals,Inc.KYPROLIS(卡非佐米 carfilzomib)注射用,用于静脉注射美国最初批准:2012
作用机制
Carfilzomib是一种四肽环氧酮蛋白酶体抑制剂,可不可逆地结合20S蛋白酶体的含N末端苏氨酸的活性位点,即26S蛋白酶体内的蛋白水解核心粒子。卡非佐米在体外固体和血液肿瘤细胞中具有抗增殖和促凋亡活性。在动物中,卡非佐米抑制血液和组织中的蛋白酶体活性,并延迟多发性骨髓瘤,血液学和实体瘤模型中的肿瘤生长。
适应症和用法
Kyprolis是一种蛋白酶体抑制剂,表明:联合地塞米松或来那度胺加地塞米松治疗复发或难治性多发性骨髓瘤患者,已接受一至三疗程治疗。作为单一药剂,用于治疗已接受一种或多种治疗的复发或难治性多发性骨髓瘤患者。剂量和给药有关剂量,请参阅完整处方信息。根据需要在Kyprolis施用之前和之后进行水合。在所有第1周期剂量之前预先给予Kyprolis输注地塞米松,并且如果输注反应症状出现或再次出现。通过30分钟输注给予20/56mg/m2regimen,通过10分钟输注给予20/27mg/m 2regimen。剂量形式和强度用于注射:10mg,30mg或60mg,在单剂量小瓶中的冻干粉末用于重构。
禁忌症
没有
警告和注意事项
心脏毒性:监测心力衰竭或缺血的体征和症状。扣留Kyprolis并及时评估。
急性肾功能衰竭:定期监测血清肌酐。
肿瘤裂解综合征(TLS):给予治疗前水合作用。监测TLS,包括尿酸水平并及时治疗。肺部毒性,包括急性呼吸窘迫综合征,急性呼吸衰竭和急性弥漫性浸润性肺病:扣留Kyprolis并及时评估。
肺动脉高压:扣留Kyprolis并进行评估。呼吸困难:对于严重或危及生命的呼吸困难,扣留Kyprolis并进行评估。
包括高血压危象在内的高血压:定期监测血压。如果高血压无法控制,请用Kyprolis中断治疗。
静脉血栓形成:建议进行血栓预防。输液反应:用地塞米松预先给药。
出血:可能发生致命或严重的出血病例,包括胃肠道,肺部和颅内出血。及时评估失血的症状和体征。
血小板减少症:监测血小板计数;按临床指示中断或减少Kyprolisdosing。
肝脏毒性和肝功能衰竭:定期监测肝脏酶。如果怀疑是Kyprolis。血栓性微血管病:监测体征和症状。如果怀疑,请停止使用Kyprolis。
后部可逆性脑病综合征(PRES):考虑发生视觉或神经症状的神经放射成像(MRI);如果怀疑,停止Kyprolis。在新诊断的移植不合格患者中,与美法仑和泼尼松联合使用会增加致命和严重毒性。
胚胎 - 胎儿毒性:Kyprolis会导致胎儿伤害。有生育能力的女性应该避免在接受治疗时怀孕。
不良反应
Kyprolis在单药治疗试验中至少有20%患者出现最常见的不良反应:贫血,疲劳,血小板减少,恶心,发热,呼吸困难,腹泻,头痛,咳嗽,外周水肿。 在联合治疗试验中,至少有20%的患者接受Kyprolis治疗,最常见的不良反应是:贫血,中性粒细胞减少,腹泻,呼吸困难,疲劳,血小板减少,发热,失眠,肌肉痉挛,咳嗽,上呼吸道感染,低钾血症。
用于特定人群老年人使用:
在Kyprolis临床试验中,≥75岁患者的不良事件发生率更高。肝功能损害:在患有轻度或中度肝功能损害的患者中,将Kyprolis的剂量减少25%。血液透析患者:在血液透析程序后给予Kyprolis治疗。
如何提供/存储和处理如何提供Kyprolis(carfilzomib)提供:
单独包装的单剂量小瓶,含有10mg卡非佐米作为白色灰白色冻干饼或粉末:NDC 76075-103-01。单独包装的单剂量小瓶,含有30mg卡非佐米作为白色托特白色冻干饼或粉末:NDC 76075-102-01。单独包装的单剂量小瓶,含有60mg卡非佐米作为白色托特白色冻干饼或粉末:NDC 76075-101-01。存储和处理未开封的小瓶应冷藏(2°C至8°C; 36°F至46°F)。 保留原包装以防光
卡非佐米冻干粉注射剂英文版说明书
Kyprolis (Carfilzomib)_Onyx PharmaceuticalsImportant Safety InformationCardiac Toxicities•New onset or worsening of pre-existing cardiac failure (e.g., congestive heart failure, pulmonary edema, decreased ejection fraction), restrictive cardiomyopathy, myocardial ischemia, and myocardial infarction including fatalities have occurred following administration of KYPROLIS. Some events occurred in patients with normal baseline ventricular function. Death due to cardiac arrest has occurred within one day of KYPROLIS administration.•Monitor patients for clinical signs or symptoms of cardiac failure or cardiac ischemia. eva luate promptly if cardiac toxicity is suspected. Withhold KYPROLIS for Grade 3 or 4 cardiac adverse events until recovery, and consider whether to restart KYPROLIS at 1 dose level reduction based on a benefit/risk assessment.•While adequate hydration is required prior to each dose in Cycle 1, monitor all patients for evidence of volume overload, especially patients at risk for cardiac failure. Adjust total fluid intake as clinically appropriate in patients with baseline cardiac failure or who are at risk for cardiac failure.•Patients ≥ 75 years, the risk of cardiac failure is increased. Patients with New York Heart Association Class III and IV heart failure, recent myocardial infarction, conduction abnormalities, angina, or arrhythmias may be at greater risk for cardiac complications and should have a comprehensive medical assessment (including blood pressure and fluid management) prior to starting treatment with KYPROLIS and remain under close follow-up.Acute Renal Failure•Cases of acute renal failure and renal insufficiency adverse events (including renal failure) have occurred in patients receiving KYPROLIS. Acute renal failure was reported more frequently in patients with advanced relapsed and refractory multiple myeloma who received KYPROLIS monotherapy. Monitor renal function with regular measurement of the serum creatinine and/or estimated creatinine clearance. Reduce or withhold dose as appropriate.Tumor Lysis Syndrome•Cases of Tumor Lysis Syndrome (TLS), including fatal outcomes, have occurred in patients receiving KYPROLIS. Patients with multiple myeloma and a high tumor burden should be considered at greater risk for TLS. Adequate hydration is required prior to each dose in Cycle 1, and in subsequent cycles as needed. Consider uric acid lowering drugs in patients at risk for TLS. Monitor for evidence of TLS during treatment and manage promptly. Withhold KYPROLIS until TLS is resolved.Pulmonary Toxicity•Acute Respiratory Distress Syndrome (ARDS), acute respiratory failure, and acute diffuse infiltrative pulmonary disease such as pneumonitis and interstitial lung disease have occurred in patients receiving KYPROLIS. Some events have been fatal. In the event of drug‐induced pulmonary toxicity, discontinue KYPROLIS.Pulmonary Hypertension•Pulmonary arterial hypertension (PAH) was reported in patients treated with KYPROLIS. eva luate with cardiac imaging and/or other tests as indicated. Withhold KYPROLIS for PAH until resolved or returned to baseline and consider whether to restart KYPROLIS based on a benefit/risk assessment.Dyspnea•Dyspnea was reported in patients treated with KYPROLIS. eva luate dyspnea to exclude cardiopulmonary conditions including cardiac failure and pulmonary syndromes. Stop KYPROLIS for Grade 3 or 4 dyspnea until resolved or returned to baseline. Consider whether to restart KYPROLIS based on a benefit/risk assessment.Hypertension•Hypertension, including hypertensive crisis and hypertensive emergency, has been observed with KYPROLIS. Some of these events have been fatal. Monitor blood pressure regularly in all patients. If hypertension cannot be adequately controlled, withhold KYPROLIS and eva luate. Consider whether to restart KYPROLIS based on a benefit/risk assessment.Venous Thrombosis•Venous thromboembolic events (including deep venous thrombosis and pulmonary embolism) have been observed with KYPROLIS. Thromboprophylaxis is recommended for patients being treated with the combination of KYPROLIS with dexamethasone or with lenalidomide plus dexamethasone. The thromboprophylaxis regimen should be based on an assessment of the patient’s underlying risks.•Patients using oral contraceptives or a hormonal method of contraception associated with a risk of thrombosis should consider an alternative method of effective contraception during treatment with KYPROLIS in combination with dexamethasone or lenalidomide plus dexamethasone.Infusion Reactions•Infusion reactions, including life‐threatening reactions, have occurred in patients receiving KYPROLIS. Symptoms include fever, chills, arthralgia, myalgia, facial flushing, facial edema, vomiting, weakness, shortness of breath, hypotension, syncope, chest tightness, or angina. These reactions can occur immediately following or up to 24 hours after administration of KYPROLIS. Premedicate with dexamethasone to reduce the incidence and severity of infusion reactions. Inform patients of the risk and of symptoms of an infusion reaction and to contact a physician immediately if they occur.Hemorrhage•Fatal or serious cases of hemorrhage have been reported in patients receiving KYPROLIS. Hemorrhagic events have included gastrointestinal, pulmonary, and intracranial hemorrhage and epistaxis. Promptly eva luate signs and symptoms of blood loss. Reduce or withhold dose as appropriate.Thrombocytopenia•KYPROLIS causes thrombocytopenia with recovery to baseline platelet count usually by the start of the next cycle. Thrombocytopenia was reported in patients receiving KYPROLIS. Monitor platelet counts frequently during treatment with KYPROLIS. Reduce or withhold dose as appropriate.Hepatic Toxicity and Hepatic Failure•Cases of hepatic failure, including fatal cases, have been reported during treatment with KYPROLIS. KYPROLIS can cause increased serum transaminases. Monitor liver enzymes regularly regardless of baseline values. Reduce or withhold dose as appropriate.Thrombotic Microangiopathy•Cases of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal outcome have occurred in patients receiving KYPROLIS. Monitor for signs and symptoms of TTP/HUS. Discontinue KYPROLIS if diagnosis is suspected. If the diagnosis of TTP/HUS is excluded, KYPROLIS may be restarted. The safety of reinitiating KYPROLIS therapy in patients previously experiencing TTP/HUS is not known.Posterior Reversible Encephalopathy Syndrome (PRES)•Cases of PRES have occurred in patients receiving KYPROLIS. PRES was formerly known as Reversible Posterior Leukoencephalopathy Syndrome. Consider a neuro‐radiological imaging (MRI) for onset of visual or neurological symptoms. Discontinue KYPROLIS if PRES is suspected and eva luate. The safety of reinitiating KYPROLIS therapy in patients previously experiencing PRES is not known.Embryo-fetal Toxicity•KYPROLIS can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals.•Females of reproductive potential should be advised to avoid becoming pregnant while being treated with KYPROLIS. Males of reproductive potential should be advised to avoid fathering a child while being treated with KYPROLIS. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the patient should be apprised of the potential hazard to the fetus.Adverse reactions•The most common adverse reactions occurring in at least 20% of patients treated with KYPROLIS in the combination therapy trials: anemia, neutropenia, diarrhea, dyspnea, fatigue, thrombocytopenia, pyrexia, insomnia, muscle spasm, cough, upper respiratory tract infection, hypokalemia.•The most common adverse reactions occurring in at least 20% of patients treated with KYPROLIS in monotherapy trials: anemia, fatigue, thrombocytopenia, nausea, pyrexia, dyspnea, diarrhea, headache, cough, edema peripheral.Please see full Prescribing Information.Indications•KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone or with lenalidomide plus dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy.•KYPROLIS® is indicated as a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.file:///C:/Users/Administrator/AppData/Local/Microsoft/Windows/Temporary%20Internet%20Files/Content.IE5/NEIQ7KY6/kyprolis_pi.pdf
药店国别:
产地国家:美国
处方药:是
所属类别: 10毫克/瓶 1瓶
包装规格: 10毫克/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:AMGEN USA INC
原产地英文商品名:KYPROLIS 10MG SDV LYO PWD 1/EA
原产地英文药品名:CARFILZOMIB
中文参考商品译名:KYPROLIS冻干粉注射剂 10毫克/瓶 1瓶
中文参考药品译名:卡非佐米
曾用名:
简介:美国FDA批准蛋白酶抑制剂carfilzomib(Kyprolis)用于接受过其他疗法但效果不佳的多发性骨髓瘤患者的治疗。批准该药用于至少两种已有疗法 (其中之一必须为硼替佐米,Velcade) 无效的复发性或难治性骨髓瘤患者的治疗。FDA的药物评价和研究中心血液学和肿瘤室主任Richard Pazdur,M.D 说:“Kyprolis的批准对尽管使用可得到的治疗其疾病已进展的多发性骨髓瘤患者提供治疗选择,”“在过去十年通过为多发性骨髓瘤药物不断的进展,提供这种疾病改善治疗。我们受到鼓舞。”批准日期2012年7月20日;公司:Onyx Pharmaceuticals,Inc.KYPROLIS(卡非佐米 carfilzomib)注射用,用于静脉注射美国最初批准:2012
作用机制
Carfilzomib是一种四肽环氧酮蛋白酶体抑制剂,可不可逆地结合20S蛋白酶体的含N末端苏氨酸的活性位点,即26S蛋白酶体内的蛋白水解核心粒子。卡非佐米在体外固体和血液肿瘤细胞中具有抗增殖和促凋亡活性。在动物中,卡非佐米抑制血液和组织中的蛋白酶体活性,并延迟多发性骨髓瘤,血液学和实体瘤模型中的肿瘤生长。
适应症和用法
Kyprolis是一种蛋白酶体抑制剂,表明:联合地塞米松或来那度胺加地塞米松治疗复发或难治性多发性骨髓瘤患者,已接受一至三疗程治疗。作为单一药剂,用于治疗已接受一种或多种治疗的复发或难治性多发性骨髓瘤患者。剂量和给药有关剂量,请参阅完整处方信息。根据需要在Kyprolis施用之前和之后进行水合。在所有第1周期剂量之前预先给予Kyprolis输注地塞米松,并且如果输注反应症状出现或再次出现。通过30分钟输注给予20/56mg/m2regimen,通过10分钟输注给予20/27mg/m 2regimen。剂量形式和强度用于注射:10mg,30mg或60mg,在单剂量小瓶中的冻干粉末用于重构。
禁忌症
没有
警告和注意事项
心脏毒性:监测心力衰竭或缺血的体征和症状。扣留Kyprolis并及时评估。
急性肾功能衰竭:定期监测血清肌酐。
肿瘤裂解综合征(TLS):给予治疗前水合作用。监测TLS,包括尿酸水平并及时治疗。肺部毒性,包括急性呼吸窘迫综合征,急性呼吸衰竭和急性弥漫性浸润性肺病:扣留Kyprolis并及时评估。
肺动脉高压:扣留Kyprolis并进行评估。呼吸困难:对于严重或危及生命的呼吸困难,扣留Kyprolis并进行评估。
包括高血压危象在内的高血压:定期监测血压。如果高血压无法控制,请用Kyprolis中断治疗。
静脉血栓形成:建议进行血栓预防。输液反应:用地塞米松预先给药。
出血:可能发生致命或严重的出血病例,包括胃肠道,肺部和颅内出血。及时评估失血的症状和体征。
血小板减少症:监测血小板计数;按临床指示中断或减少Kyprolisdosing。
肝脏毒性和肝功能衰竭:定期监测肝脏酶。如果怀疑是Kyprolis。血栓性微血管病:监测体征和症状。如果怀疑,请停止使用Kyprolis。
后部可逆性脑病综合征(PRES):考虑发生视觉或神经症状的神经放射成像(MRI);如果怀疑,停止Kyprolis。在新诊断的移植不合格患者中,与美法仑和泼尼松联合使用会增加致命和严重毒性。
胚胎 - 胎儿毒性:Kyprolis会导致胎儿伤害。有生育能力的女性应该避免在接受治疗时怀孕。
不良反应
Kyprolis在单药治疗试验中至少有20%患者出现最常见的不良反应:贫血,疲劳,血小板减少,恶心,发热,呼吸困难,腹泻,头痛,咳嗽,外周水肿。 在联合治疗试验中,至少有20%的患者接受Kyprolis治疗,最常见的不良反应是:贫血,中性粒细胞减少,腹泻,呼吸困难,疲劳,血小板减少,发热,失眠,肌肉痉挛,咳嗽,上呼吸道感染,低钾血症。
用于特定人群老年人使用:
在Kyprolis临床试验中,≥75岁患者的不良事件发生率更高。肝功能损害:在患有轻度或中度肝功能损害的患者中,将Kyprolis的剂量减少25%。血液透析患者:在血液透析程序后给予Kyprolis治疗。
如何提供/存储和处理如何提供Kyprolis(carfilzomib)提供:
单独包装的单剂量小瓶,含有10mg卡非佐米作为白色灰白色冻干饼或粉末:NDC 76075-103-01。单独包装的单剂量小瓶,含有30mg卡非佐米作为白色托特白色冻干饼或粉末:NDC 76075-102-01。单独包装的单剂量小瓶,含有60mg卡非佐米作为白色托特白色冻干饼或粉末:NDC 76075-101-01。存储和处理未开封的小瓶应冷藏(2°C至8°C; 36°F至46°F)。 保留原包装以防光
卡非佐米冻干粉注射剂英文版说明书
Kyprolis (Carfilzomib)_Onyx PharmaceuticalsImportant Safety InformationCardiac Toxicities•New onset or worsening of pre-existing cardiac failure (e.g., congestive heart failure, pulmonary edema, decreased ejection fraction), restrictive cardiomyopathy, myocardial ischemia, and myocardial infarction including fatalities have occurred following administration of KYPROLIS. Some events occurred in patients with normal baseline ventricular function. Death due to cardiac arrest has occurred within one day of KYPROLIS administration.•Monitor patients for clinical signs or symptoms of cardiac failure or cardiac ischemia. eva luate promptly if cardiac toxicity is suspected. Withhold KYPROLIS for Grade 3 or 4 cardiac adverse events until recovery, and consider whether to restart KYPROLIS at 1 dose level reduction based on a benefit/risk assessment.•While adequate hydration is required prior to each dose in Cycle 1, monitor all patients for evidence of volume overload, especially patients at risk for cardiac failure. Adjust total fluid intake as clinically appropriate in patients with baseline cardiac failure or who are at risk for cardiac failure.•Patients ≥ 75 years, the risk of cardiac failure is increased. Patients with New York Heart Association Class III and IV heart failure, recent myocardial infarction, conduction abnormalities, angina, or arrhythmias may be at greater risk for cardiac complications and should have a comprehensive medical assessment (including blood pressure and fluid management) prior to starting treatment with KYPROLIS and remain under close follow-up.Acute Renal Failure•Cases of acute renal failure and renal insufficiency adverse events (including renal failure) have occurred in patients receiving KYPROLIS. Acute renal failure was reported more frequently in patients with advanced relapsed and refractory multiple myeloma who received KYPROLIS monotherapy. Monitor renal function with regular measurement of the serum creatinine and/or estimated creatinine clearance. Reduce or withhold dose as appropriate.Tumor Lysis Syndrome•Cases of Tumor Lysis Syndrome (TLS), including fatal outcomes, have occurred in patients receiving KYPROLIS. Patients with multiple myeloma and a high tumor burden should be considered at greater risk for TLS. Adequate hydration is required prior to each dose in Cycle 1, and in subsequent cycles as needed. Consider uric acid lowering drugs in patients at risk for TLS. Monitor for evidence of TLS during treatment and manage promptly. Withhold KYPROLIS until TLS is resolved.Pulmonary Toxicity•Acute Respiratory Distress Syndrome (ARDS), acute respiratory failure, and acute diffuse infiltrative pulmonary disease such as pneumonitis and interstitial lung disease have occurred in patients receiving KYPROLIS. Some events have been fatal. In the event of drug‐induced pulmonary toxicity, discontinue KYPROLIS.Pulmonary Hypertension•Pulmonary arterial hypertension (PAH) was reported in patients treated with KYPROLIS. eva luate with cardiac imaging and/or other tests as indicated. Withhold KYPROLIS for PAH until resolved or returned to baseline and consider whether to restart KYPROLIS based on a benefit/risk assessment.Dyspnea•Dyspnea was reported in patients treated with KYPROLIS. eva luate dyspnea to exclude cardiopulmonary conditions including cardiac failure and pulmonary syndromes. Stop KYPROLIS for Grade 3 or 4 dyspnea until resolved or returned to baseline. Consider whether to restart KYPROLIS based on a benefit/risk assessment.Hypertension•Hypertension, including hypertensive crisis and hypertensive emergency, has been observed with KYPROLIS. Some of these events have been fatal. Monitor blood pressure regularly in all patients. If hypertension cannot be adequately controlled, withhold KYPROLIS and eva luate. Consider whether to restart KYPROLIS based on a benefit/risk assessment.Venous Thrombosis•Venous thromboembolic events (including deep venous thrombosis and pulmonary embolism) have been observed with KYPROLIS. Thromboprophylaxis is recommended for patients being treated with the combination of KYPROLIS with dexamethasone or with lenalidomide plus dexamethasone. The thromboprophylaxis regimen should be based on an assessment of the patient’s underlying risks.•Patients using oral contraceptives or a hormonal method of contraception associated with a risk of thrombosis should consider an alternative method of effective contraception during treatment with KYPROLIS in combination with dexamethasone or lenalidomide plus dexamethasone.Infusion Reactions•Infusion reactions, including life‐threatening reactions, have occurred in patients receiving KYPROLIS. Symptoms include fever, chills, arthralgia, myalgia, facial flushing, facial edema, vomiting, weakness, shortness of breath, hypotension, syncope, chest tightness, or angina. These reactions can occur immediately following or up to 24 hours after administration of KYPROLIS. Premedicate with dexamethasone to reduce the incidence and severity of infusion reactions. Inform patients of the risk and of symptoms of an infusion reaction and to contact a physician immediately if they occur.Hemorrhage•Fatal or serious cases of hemorrhage have been reported in patients receiving KYPROLIS. Hemorrhagic events have included gastrointestinal, pulmonary, and intracranial hemorrhage and epistaxis. Promptly eva luate signs and symptoms of blood loss. Reduce or withhold dose as appropriate.Thrombocytopenia•KYPROLIS causes thrombocytopenia with recovery to baseline platelet count usually by the start of the next cycle. Thrombocytopenia was reported in patients receiving KYPROLIS. Monitor platelet counts frequently during treatment with KYPROLIS. Reduce or withhold dose as appropriate.Hepatic Toxicity and Hepatic Failure•Cases of hepatic failure, including fatal cases, have been reported during treatment with KYPROLIS. KYPROLIS can cause increased serum transaminases. Monitor liver enzymes regularly regardless of baseline values. Reduce or withhold dose as appropriate.Thrombotic Microangiopathy•Cases of thrombotic microangiopathy, including thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS), including fatal outcome have occurred in patients receiving KYPROLIS. Monitor for signs and symptoms of TTP/HUS. Discontinue KYPROLIS if diagnosis is suspected. If the diagnosis of TTP/HUS is excluded, KYPROLIS may be restarted. The safety of reinitiating KYPROLIS therapy in patients previously experiencing TTP/HUS is not known.Posterior Reversible Encephalopathy Syndrome (PRES)•Cases of PRES have occurred in patients receiving KYPROLIS. PRES was formerly known as Reversible Posterior Leukoencephalopathy Syndrome. Consider a neuro‐radiological imaging (MRI) for onset of visual or neurological symptoms. Discontinue KYPROLIS if PRES is suspected and eva luate. The safety of reinitiating KYPROLIS therapy in patients previously experiencing PRES is not known.Embryo-fetal Toxicity•KYPROLIS can cause fetal harm when administered to a pregnant woman based on its mechanism of action and findings in animals.•Females of reproductive potential should be advised to avoid becoming pregnant while being treated with KYPROLIS. Males of reproductive potential should be advised to avoid fathering a child while being treated with KYPROLIS. If this drug is used during pregnancy, or if pregnancy occurs while taking this drug, the patient should be apprised of the potential hazard to the fetus.Adverse reactions•The most common adverse reactions occurring in at least 20% of patients treated with KYPROLIS in the combination therapy trials: anemia, neutropenia, diarrhea, dyspnea, fatigue, thrombocytopenia, pyrexia, insomnia, muscle spasm, cough, upper respiratory tract infection, hypokalemia.•The most common adverse reactions occurring in at least 20% of patients treated with KYPROLIS in monotherapy trials: anemia, fatigue, thrombocytopenia, nausea, pyrexia, dyspnea, diarrhea, headache, cough, edema peripheral.Please see full Prescribing Information.Indications•KYPROLIS® (carfilzomib) is indicated in combination with dexamethasone or with lenalidomide plus dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma who have received one to three lines of therapy.•KYPROLIS® is indicated as a single agent for the treatment of patients with relapsed or refractory multiple myeloma who have received one or more lines of therapy.file:///C:/Users/Administrator/AppData/Local/Microsoft/Windows/Temporary%20Internet%20Files/Content.IE5/NEIQ7KY6/kyprolis_pi.pdf
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡非佐米冻干粉注射剂Kyprolis Injection 10mg(carfilzomib)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡非佐米冻干粉注射剂Kyprolis Injection 10mg(carfilzomib)