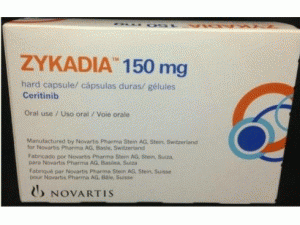
色瑞替尼,色瑞替尼硬胶囊ceritinib(Zykadia 150mg hard capsules)
 药店国别:无
产地国家:英国
处方药:是
所属类别: 150毫克/粒 90粒/盒
包装规格: 150毫克/粒 90粒/盒
计价单位:盒
生产厂家中文参考译名:无
生产厂家英文名:Novartis Pharmaceuticals UK Ltd
原产地英文商品名:Zykadia 150mg hard capsules 90capsules
原产地英文药品名:ceritinib
中文参考商品译名:Zykadia胶囊 150毫克/粒 90粒/盒
中文参考药品译名:色瑞替尼
曾用名:LDK378
药店国别:无
产地国家:英国
处方药:是
所属类别: 150毫克/粒 90粒/盒
包装规格: 150毫克/粒 90粒/盒
计价单位:盒
生产厂家中文参考译名:无
生产厂家英文名:Novartis Pharmaceuticals UK Ltd
原产地英文商品名:Zykadia 150mg hard capsules 90capsules
原产地英文药品名:ceritinib
中文参考商品译名:Zykadia胶囊 150毫克/粒 90粒/盒
中文参考药品译名:色瑞替尼
曾用名:LDK378
简介
部份中文色瑞替尼处方资料(仅供参考) 商品名:Zykadia® 英文名:Ceritinib Capsules 中文名:色瑞替尼 作用机制 Ceritinib是一种激酶抑制剂。在临床相关浓度的生物化学或细胞测定中鉴定的ceritinib抑制的靶标包括ALK,胰岛素样生长因子1受体(IGF-1R),胰岛素受体(InsR)和ROS1。 其中,ceritinib对ALK的活性最高。在体外和体内测定中,Ceritinib抑制ALK的自身磷酸化,ALK介导的下游信号蛋白STAT3的磷酸化和ALK依赖性癌细胞的增殖。 Ceritinib抑制表达EML4-ALK和NPM-ALK融合蛋白的细胞系的体外增殖,并证实在小鼠和大鼠中EML4-ALK阳性NSCLC异种移植物生长的剂量依赖性抑制。Ceritinib在携带EML4-ALK阳性NSCLC异种移植物的小鼠中表现出剂量依赖性抗肿瘤活性,其在临床相关范围内的浓度下显示出对克唑替尼的抗性。 适应证和用途 色瑞替尼是一种激酶抑制剂适用为对克唑替尼[crizotinib]治疗后已进展或不能耐受的间变性淋巴瘤激酶(ALK)-阳性转移非小细胞肺癌(NSCLC)患者的治疗。这个是一种是在根据肿瘤反应率和反应时间在加速批准下被批准的。尚未确定生存或疾病-相关症状改善。可能依验证性试验临床获益证实和描述情况而确定继续批准这个适应证。 剂量和给药方法 每天1次口服750mg。空腹给予ZYKADIA(即,不要餐后2小时内给予)。 剂型和规格胶囊:150mg 禁忌证:无 警告和注意事项 ⑴ 严重和持续胃肠道毒性:在38%患者由于发生腹泻,恶心,呕吐或腹痛调整剂量。如止抗吐药或止泻药无反应不给药,然后减低ZYKADIA剂量。 ⑵ 肝毒性:ZYKADIA可能致肝毒性。至少每月监查肝实验室检验。不给药然后减低剂量,或永久终止ZYKADIA。 ⑶ 间质性肺疾病(ILD)/肺炎:在4%患者中发生。在被诊断有治疗相关ILD/肺炎患者中永久终止ZYKADIA。 ⑷QT间期延长:ZYKADIA可能致QTc间期延长。监视心电图和电解质in患者有充血性心脏衰竭,缓慢性心律失常,电解质异常,或患者正在用药物已知延长QTc间期。不给药然后减低剂量,或永久终止ZYKADIA。 ⑸ 高血糖:ZYKADIA可能致高血糖。监视葡萄糖和如指示开始或优化抗高血糖药物。不给药然后减低剂量,或永久终止ZYKADIA。 ⑹ 心动过缓:ZYKADIA可能致心动过缓。定期监视心率和血压。不给药然后减低剂量,或永久终止ZYKADIA。 ⑺ 胚胎胎儿毒性:ZYKADIA可能致胎儿危害。忠告有生殖潜能女性对胎儿潜在风险。 不良反应 最常见不良反应(发生率至少25%)为腹泻,恶心,转氨酶升高,呕吐,腹痛,疲乏,食欲减退,和便秘。 药物相互作用 ⑴ CYP3A抑制剂和诱导剂:避免ZYKADIA与强CYP3A抑制剂或诱导剂的同时使用。如不可避免同时使用某种强CYP3A抑制剂,减低ZYKADIA的剂量。 ⑵ CYP3A和CYP2C9底物:避免ZYKADIA 与有狭窄治疗指数的CYP3A或CYP2C9底物同时使用。英文版说明
Zykadia 150mg hard capsules 90capsulesDescription:ZYKADIA (ceritinib) is a tyrosine kinase inhibitor for oral administration. The molecular formula for ceritinib is C28H36N5O3ClS. The molecular weight is 558.14 g/mole. Ceritinib is described chemically as 5-Chloro-N4-[2-[(1-methylethyl)sulfonyl]phenyl]-N2-[5-methyl-2-(1-methylethoxy)-4-(4-piperidinyl)phenyl]-2,4-pyrimidinediamine.Ceritinib is a white to almost white or light yellow or light brown powder with a pKa of 9.7 and 4.1.ZYKADIA is supplied as printed hard-gelatin capsules containing 150 mg of ceritinib and the following inactive ingredients: colloidal anhydrous silica, L-hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and hard gelatin capsule shells. The capsule shell is composed of gelatin, indiogotine, and titanium dioxide.CLINICAL PHARMACOLOGY:Mechanism of Action:Ceritinib is a kinase inhibitor. Targets of ceritinib inhibition identified in either biochemical or cellular assays at clinically relevant concentrations include ALK, insulin-like growth factor 1 receptor (IGF-1R), insulin receptor (InsR), and ROS1. Among these, ceritinib is most active against ALK. Ceritinib inhibited autophosphorylation of ALK, ALK-mediated phosphorylation of the downstream signaling protein STAT3, and proliferation of ALK-dependent cancer cells in in vitro and in vivo assays.Ceritinib inhibited the in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice and rats. Ceritinib exhibited dose-dependent anti-tumor activity in mice bearing EML4-ALK-positive NSCLC xenografts with demonstrated resistance to crizotinib, at concentrations within a clinically relevant range.INDICATIONS AND USAGE:ZYKADIA is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies - package insert]. An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.USE IN SPECIFIC POPULATIONSPregnancyPregnancy Category DRisk SummaryBased on its mechanism of action, ZYKADIA may cause fetal harm when administered to a pregnant woman. In animal studies, administration of ceritinib to rats and rabbits during organogenesis at maternal plasma exposures below the recommended human dose caused increases in skeletal anomalies in rats and rabbits. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus.Animal DataIn an embryo-fetal development study in which pregnant rats were administered daily doses of ceritinib during organogenesis, dose-related skeletal anomalies were observed at doses as low as 50 mg/kg (less than 0.5-fold the human exposure by AUC at the recommended dose). Findings included delayed ossifications and skeletal variations.In pregnant rabbits administered ceritinib daily during organogenesis, dose-related skeletal anomalies, including incomplete ossification, were observed at doses equal to or greater than 2 mg/kg/day (approximately 0.015-fold the human exposure by AUC at the recommended dose). A low incidence of visceral anomalies, including absent or malpositioned gallbladder and retroesophageal subclavian cardiac artery, was observed at doses equal to or greater than 10 mg/kg/day (approximately 0.13-fold the human exposure by AUC at the recommended dose). Maternal toxicity and abortion occurred in rabbits at doses of 35 mg/kg or greater. In addition, embryolethality was observed in rabbits at a dose of 50 mg/kg.Nursing MothersIt is not known whether ceritinib or its metabolites are present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from ceritinib, advise mothers to discontinue nursing.Pediatric UseThe safety and effectiveness of ZYKADIA in pediatric patients have not been established.Geriatric UseClinical studies of ZYKADIA did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Of the 255 patients in Study 1 who received ZYKADIA at the recommended dose, 40 (16%) were 65 years or older.Hepatic ImpairmentAs ceritinib is eliminated primarily via the liver, patients with hepatic impairment may have increased exposure. Dose adjustment is not recommended for patients with mild hepatic impairment (total bilirubin less than or equal to ULN and AST greater than ULN or total bilirubin greater than 1.0 to 1.5 times ULN andhttps://www.medicines.org.uk/emc/product/7109用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 色瑞替尼,色瑞替尼硬胶囊ceritinib(Zykadia 150mg hard capsules)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 色瑞替尼,色瑞替尼硬胶囊ceritinib(Zykadia 150mg hard capsules)