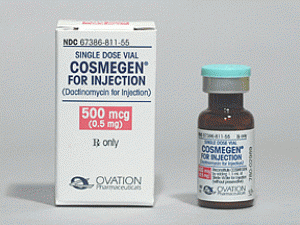
更生霉素放线菌素d注射剂COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)
 药店国别:
产地国家:美国
处方药:是
所属类别: 0.5毫克/瓶 1瓶
包装规格: 0.5毫克/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:ASD/RECORDATI
原产地英文商品名:COSMEGEN VL 0.5MG ASD DS 1
原产地英文药品名:DACTINOMYCIN
中文参考商品译名:COSMEGEN注射剂 0.5毫克/瓶 1瓶
中文参考药品译名:更生霉素放线菌素
曾用名:
药店国别:
产地国家:美国
处方药:是
所属类别: 0.5毫克/瓶 1瓶
包装规格: 0.5毫克/瓶 1瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:ASD/RECORDATI
原产地英文商品名:COSMEGEN VL 0.5MG ASD DS 1
原产地英文药品名:DACTINOMYCIN
中文参考商品译名:COSMEGEN注射剂 0.5毫克/瓶 1瓶
中文参考药品译名:更生霉素放线菌素
曾用名:
简介
部份中文更生霉素放线菌素处方资料(仅供参考) 商品名:COSMEGEN0.5MG(Dactinomycin) 中文名:可美净(更生霉素放线菌素d冻晶注射剂) 成份:Dactinomycin 药理类别:抗癌药物 孕妇用药分级 D级:在对照的人体研究试验中显示该药物对胚胎有不良影响,若此药能带来之效益远超过其它药物的使用,因此即使在其危险性的存在下,仍可接受此药物用于怀孕妇女上。 结构式:2-amino-N,N'-bis[(6S,9R,10S,13R,18aS)- 6,13-diisopropyl- 2,5,9-trimethyl-1,4,7,11,14-pentaoxohexadecahydro- 1H-pyrrolo[2,1-i] oxatetraazacyclohexadecin-10-yl]- 4,6-dimethyl- 3-oxo- 3H-phenoxazine- 1,9-dicarboxamideUpToDate UpToDate 连结药理作用 COSMEGEN® (dactinomycin) 为actinomycin类抗生素的一种,这类抗生素係由各个不同种的链霉菌属(streptomyces) 所产生。Dactinomycin是Streptomycesparvullus所产生的actinomycin类抗生素混合物中的主要成份,由于actinomycin类抗生素的抗菌活性伴随而来的毒性,使它不能用于感染性疾病的治疗,然而对实验动物各类型植入肿瘤的研究证实具有抗肿瘤作用,这种细胞毒性作用可用于治疗某些癌症。适应症小儿癌、睪丸恶性肿瘤。 用法用量 成人: 通常成人的剂量是每日静脉注射500mcg(0.5mg),最多使用五天。 孩童: 通常孩童每日每公斤体重静脉注射15mcg(0.015),使用五天。另一个疗程是为期一週静脉注射总计量为体表面积每平方公尺2,500mcg(2.5mg)。成人及孩童必须要经过叁週以后,在所有药物毒性的徵候都消失的前提下,才能开始第二个疗程。 更生霉素放线菌素d注射剂英文版说明书 Cosmegen (Dactinomycin)COSMEGEN®MSDDactinomycinAntineoplasticAction And Clinical Pharmacology: Dactinomycin is an antibiotic obtained as the principal component of the mixture of actinomycins produced by S. parvullus. This organism, unlike other species, yields an essentially pure substance that contains only traces of similar compounds differing in the amino acid content of the peptide side chains. It is available for clinical use as a sterile, yellow lyophilized powder which, in aqueous solution, produces a clear gold color.Generally, the actinomycins exert an inhibitory effect on gram positive and gram negative bacteria and on some fungi. However, the toxic properties of the actinomycins (including dactinomycin) in relation to antibacterial activity are such as to preclude their use as antibiotics in the treatment of infectious diseases.Because the actinomycins are cytotoxic, they have an antineoplastic effect which has been demonstrated in experimental animals with various types of tumor implant. This cytotoxic action is the basis for their use in the palliative treatment of certain types of cancer.Pharmacokinetics: Results of a study in patients with malignant melanoma indicate that dactinomycin ( actinomycin D) is minimally metabolized, is concentrated in nucleated cells, and does not penetrate the blood brain barrier. Approximately 30% of the dose was recovered in urine and feces in one week. The terminal plasma half-life for radioactivity was approximately 36 hours.Indications And Clinical Uses: Wilms’ Tumor: The neoplasm responding most frequently to dactinomycin is Wilms’ tumor. With low doses of both dactinomycin and radiotherapy, temporary objective improvement may be as good as and may last longer than with higher doses of each given alone. In the National Wilms’ Tumor study, combination therapy with dactinomycin and vincristine together with surgery and radiotherapy, was shown to have significantly improved the prognosis of patients in groups II and III. Dactinomycin and vincristine were given for a total of seven cycles, so that maintenance therapy continued for approximately 15 months.Postoperative radiotherapy in group I patients and optimal combination chemotherapy for those in group IV are unsettled issues. About 70% of lung metastases have disappeared with an appropriate combination of radiation, dactinomycin and vincristine.Rhabdomyosarcoma: Temporary regression of the tumor and beneficial subjective results have occurred with dactinomycin in rhabdomyosarcoma which, like most soft tissue sarcomas, is comparatively radioresistant.Several groups have reported successful use of cyclophosphamide, vincristine, dactinomycin and doxorubicin hydrochloride in various combinations. Effective combinations have included vincristine and dactinomycin; vincristine, dactinomycin and cyclophosphamide (VAC therapy) and all 4 drugs in sequence. At present, the most effective treatment for children with inoperable or metastatic rhabdomyosarcoma has been VAC chemotherapy. Two-thirds of these children were doing well without evidence of disease at a median time of three years after diagnosis.Carcinoma of Testis and Uterus: The sequential use of dactinomycin and methotrexate, along with meticulous monitoring of human chorionic gonadotropin levels until normal, has resulted in survival in the majority of women with metastatic choriocarcinoma. Sequential therapy is used if there is: stability in gonadotropin titers following two successive courses of an agent; rising gonadotropin titers during treatment; severe toxicity preventing adequate therapy.In patients with nonmetastatic choriocarcinoma, dactinomycin or methotrexate or both have been used successfully, with or without surgery.Dactinomycin has been beneficial as a single agent in the treatment of metastatic non-seminomatour testicular carcinoma when used in cycles of 500 µg/day for 5 consecutive days, every 6 to 8 weeks for periods of four months or longer.Other Neoplasms: Dactinomycin has been given i.v. or by regional perfusion, either alone or with other antineoplastic compounds or x-ray therapy, in the palliative treatment of Ewing’s sarcoma and sarcoma botryoides. For non-metastatic Ewing’s sarcoma, promising results were obtained when dactinomycin (45 µg/m and cyclophosphamide (1 200 mg/m were given sequentially and with radiotherapy, over an 18 month period. Those with metastatic disease remain the subject of continued investigation with a more aggressive chemotherapeutic regimen employed initially.Temporary objective improvement and relief of pain and discomfort have followed the use of dactinomycin usually in conjunction with radiotherapy for sarcoma botryoides. This palliative effect ranges from transitory inhibition of tumor growth to a considerable but temporary regression in tumor size.Dactinomycin and Radiation Therapy: Much evidence suggests that dactinomycin potentiates the effects of x-ray therapy. The converse also appears likely; i.e., dactinomycin may be more effective when radiation therapy also is given.With combined dactinomycin-radiation therapy, the normal skin, as well as the buccal and pharyngeal mucosa, show early erythema. A smaller than usual x-ray dose when given with dactinomycin causes erythema and vesiculation, which progress more rapidly through the stages of tanning and desquamation. Healing may occur in 4 to 6 weeks rather than 2 to 3 months. Erythema from previous x-ray therapy may be reactivated by dactinomycin alone, even when irradiation occurred many months earlier, and especially when the interval between the two forms of therapy is brief. This potentiation of radiation effect represents a special problem when the irradiation treatment area includes the mucous membrane. When irradiation is directed toward the nasopharynx, the combination may produce severe oropharyngeal mucositis. Severe reactions may ensue if high doses of both dactinomycin and radiation therapy are used or if the patient is particularly sensitive to such combined therapy.Because of this potentiating effect, dactinomycin may be tried in radio-sensitive tumors not responding to doses of x-ray therapy that can be tolerated. Objective improvement in tumor size and activity may be observed when lower, better tolerated doses of both types of therapy are employed.Dactinomycin and Perfusion Technique: Dactinomycin alone or with other antineoplastic agents has also been given by the isolation-perfusion technique, either as palliative treatment or as an adjunct to resection of a tumor. Some tumors considered resistant to chemotherapy and radiation therapy may respond when the drug is given by the perfusion technique. Neoplasms in which dactinomycin has been tried by this technique include various types of sarcoma, carcinoma, and adenocarcinoma.In some instances tumors regressed, pain was relieved for variable periods, and surgery made possible. On other occasions, however, the outcome has been less favorable. Nevertheless, in selected cases, the drug by perfusion may provide more effective palliation than when given systemically.Dactinomycin by the isolation-perfusion technique offers certain advantages, provided leakage of the drug through the general circulation into other areas of the body is minimal. By this technique the drug is in continuous contact with the tumor for the duration of treatment. The dose may be increased well over that used by the systemic route, usually without adding to the danger of toxic effects. If the agent is confined to an isolated part, it should not interfere with the patient’s defense mechanism. Systemic absorption of toxic products from neoplastic tissue can be minimized by removing the perfusate when the procedure is finished.Contra-Indications: If dactinomycin is given at or about the time of infection with chickenpox or herpes zoster, a severe generalized disease, which may result in death, may occur.Precautions: General: Dactinomycin should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents.This drug is highly toxic and both powder and solution must be handled and administered with care. Inhalation of dust or vapors and contact with skin or mucous membranes, especially those of the eyes, must be avoided. Should accidental eye contact occur, copious irrigation with water should be instituted immediately, followed by prompt ophthalmologic consultation. Should accidental skin contact occur, the affected part must be irrigated immediately with copious amounts of water for at least 15 minutes.As with all antineoplastic agents, dactinomycin is a toxic drug and very careful and frequent observation of the patient for adverse reactions is necessary. These reactions may involve any tissue of the body. The possibility of an anaphylactoid reaction should be borne in mind.Increased incidence of gastrointestinal toxicity and marrow suppression has been reported when dactinomycin was given with x-ray therapy.Particular caution is necessary when administering dactinomycin in the first 2 months after irradiation for the treatment of right sided Wilms’ tumor, since hepatomegaly and elevated AST levels have been noted.Nausea and vomiting due to dactinomycin make it necessary to give this drug intermittently. It is extremely important to observe the patient daily for toxic side effects when multiple chemotherapy is employed, since a full course of therapy occasionally is not tolerated. If stomatitis, diarrhea, or severe hemopoietic depression appear during therapy, these drugs should be discontinued until the patient has recovered.Recent reports indicate an increased incidence of second primary tumors following treatment with radiation and antineoplastic agents, such as dactinomycin. Multi-modal therapy creates the need for careful, long-term observation of cancer survivors.Laboratory Tests: Many abnormalities of renal, hepatic, and bone marrow function have been reported in patients with neoplastic disease and receiving dactinomycin. It is advisable to check renal, hepatic, and bone marrow functions frequently.Drug/Laboratory Test Interactions : It has been reported that dactinomycin may interfere with bioassay procedures for the determination of antibacterial drug levels.Carcinogenesis, Mutagenesis, Impairment of Fertility: The International Agency on Research on Cancer has judged that dactinomycin is a positive carcinogen in animals. Local sarcomas were produced in mice and rats after repeated s.c. or intraperitoneal injection. Mesenchymal tumors occurred in male F344 rats given intraperitoneal injections of 0.05 mg/kg, 2 to 5 times/week for 18 weeks. The first tumor appeared at 23 weeks.Dactinomycin has been shown to be mutagenic in a number of test systems in vitro and in vivo including human fibroblasts and leucocytes, and HELA cells. DNA damage and cytogenetic effects have been demonstrated in the mouse and the rat.Adequate fertility studies have not been reported.Pregnancy: Dactinomycin has been shown to cause malformations and embryotoxicity in the rat, rabbit and hamster when given in doses of 50 to 100 µg/kg i.v. (3 to 7 times the maximum recommended human dose). There are no adequate and well-controlled studies in pregnant women. Dactinomycin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.Lactation: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from dactinomycin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Children: The greater frequency of toxic effects of dactinomycin in infants suggests that this drug should be given to infants only over the age of 6 to 12 months.Adverse Reactions: Toxic effects (excepting nausea and vomiting) usually do not become apparent until 2 to 4 days after a course of therapy is stopped, and may not be maximal before 1 to 2 weeks have elapsed. Deaths have been reported. However, adverse effects are usually reversible on discontinuance of therapy. They include the following:Miscellaneous: malaise, fatigue, lethargy, fever, myalgia, proctitis, hypocalcemia.Oral: cheilitis, dysphagia, esophagitis, ulcerative stomatitis, pharyngitis.Gastrointestinal: anorexia, nausea, vomiting, abdominal pain, diarrhea, gastrointestinal ulceration, liver toxicity including ascites, hepatomegaly, hepatitis, and liver function test abnormalities. Nausea and vomiting, which occur early during the first few hours after administration, may be alleviated by giving antiemetics.Hematologic: anemia, even to the point of aplastic anemia, agranulocytosis, leukopenia, thrombopenia, pancytopenia, reticulopenia. Platelet and white cell counts should be done daily to detect severe hemopoietic depression. If either count markedly decreases, the drug should be withheld until marrow recovery occurs (often up to 3 weeks).Dermatologic: alopecia, skin eruptions, acne, flare up of erythema or increased pigmentation of previously irradiated skin.Soft tissues: Dactinomycin is extremely corrosive. If extravasation occurs during i.v. use, severe damage to soft tissues will occur. In at least one instance, this has led to contracture of the arms.Dosage And Administration: Toxic reactions due to dactinomycin are frequent and may be severe (see Adverse Effects), thus limiting in many instances the amount that may be given. However, the severity of toxicity varies markedly and is only partly dependent on the dose employed. The drug must be given in short courses.I.V.: The dosage of dactinomycin varies depending on the tolerance of the patient, the size and location of the neoplasm, and the use of other forms of therapy. It may be necessary to decrease the usual dosages suggested below when other chemotherapy or x-ray therapy is used concomitantly or has been used previously.The dosage for adults or children should not exceed 15 g/kg or 400 to 600 g/mof body surface daily i.v. for 5 days. Calculation of the dosage for obese or edematous patients should be on the basis of surface area in an effort to relate dosage to lean body mass.Adults: The usual adult dosage is 500 µg (0.5 mg) daily i.v. for a maximum of 5 days.Children: In children 15 µg (0.015 mg)/kg of body weight is given i.v. daily for 5 days. An alternative schedule is a total dosage of 2 500 µg (2.5 mg)/mof body surface given i.v. over a 1-week period.In both adults and children, a second course may be given after at least 3 weeks have elapsed, provided all signs of toxicity have disappeared.Reconstitute dactinomycin by adding 1.1 mL of Sterile Water for Injection (without preservative) using aseptic precautions. The resulting solution of dactinomycin will contain approximately 500 g or 0.5 mg/mL.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. When reconstituted, dactinomycin is a clear, gold-colored solution.Once reconstituted, the solution of dactinomycin can be added to infusion solutions of Dextrose Injection 5% or Sodium Chloride Injection either directly or to the tubing of a running i.v. infusion.Although reconstituted dactinomycin is chemically stable, the product does not contain a preservative and accidental microbial contamination might result. Any unused portion should be discarded. Use of water containing preservatives (benzyl alcohol or parabens) to reconstitute dactinomycin for injection, results in the formation of a precipitate.Partial removal of dactinomycin from i.v. solutions by cellulose ester membrane filters used in some i.v. in-line filters has been reported.Since dactinomycin is extremely corrosive to soft tissue, precautions for materials of this nature should be observed.If the drug is given directly into the vein without the use of an infusion, the two-needle technique should be used. Reconstitute and withdraw the calculated dose from the vial with one sterile needle. Use another sterile needle for direct injection into the vein.Discard any unused portion of the dactinomycin solution.Isolation-Perfusion Technique: The dosage schedules and the technique itself vary from one investigator to another; the published literature, therefore, should be consulted for details. In general, the following doses are suggested: 50 g (0.05 mg)/kg of body weight for lower extremity or pelvis. 35 g (0.035 mg)/kg of body weight for upper extremity.It may be advisable to use lower doses in obese patients, or when previous chemotherapy or radiation therapy has been employed.Complications of the perfusion technique are related mainly to the amount of drug that escapes into the systemic circulation and may consist of hemopoietic depression, absorption of toxic products from massive destruction of neoplastic tissue, increased susceptibility to infection, impaired wound healing, and superficial ulceration of the gastric mucosa. Other side effects may include edema of the extremity involved, damage to soft tissues of the perfused area, and (potentially) venous thrombosis.Special Handling: Due to the drug’s toxic and mutagenic properties, appropriate precautions including the use of appropriate safety equipment are recommended for the preparation of dactinomycin for parenteral administration. The National Institutes of Health presently recommend that the preparation of injectable antineoplastic drugs should be performed in a Class II laminar flow biological safety cabinet and that personnel preparing drugs of this class should wear surgical gloves and a closed front surgical-type gown with knit cuffs.Availability And Storage: Each vial contains: lyophilized, amorphous yellow dactinomycin powder 500 g and mannitol 20 mg. Forms a clear gold-colored solution on reconstitution. Protect from light.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 更生霉素放线菌素d注射剂COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 更生霉素放线菌素d注射剂COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)