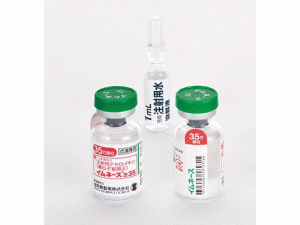
米铂冻干粉注射剂Miripla 70mg vial(Miriplatin Hydrate)
 药店国别:
产地国家:日本
处方药:是
所属类别: 70毫克/瓶
包装规格: 70毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Dainihon Sumitomo
原产地英文商品名:Miripla(ミリプラ動注用)70mg/vial 1vial
原产地英文药品名:Miriplatin Hydrate
中文参考商品译名:Miripla(ミリプラ動注用)70毫克/瓶 1瓶
中文参考药品译名:米铂
药店国别:
产地国家:日本
处方药:是
所属类别: 70毫克/瓶
包装规格: 70毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Dainihon Sumitomo
原产地英文商品名:Miripla(ミリプラ動注用)70mg/vial 1vial
原产地英文药品名:Miriplatin Hydrate
中文参考商品译名:Miripla(ミリプラ動注用)70毫克/瓶 1瓶
中文参考药品译名:米铂
简介
部分中文Miripla处方资料(仅供参考) 商品药名:Miripla® 英文药名:Miriplatin Hydrate 中文药名:米铂冻干粉注射剂 日文药名:ミリプラ動注用70mg 生产厂家:日本住友制药 药品介绍 Miriplatin Hydrate是日本住友制药株式会社开发的脂溶性铂复合物抗癌药,于2009年10月16日获得日本厚生劳动省批准,用于治疗肝细胞癌。本品专用混悬液已于同年8月20日获得批准。2010年1月20日,Miriplatin Hydrate及其专用混悬液同时上市。 药物分类名称:肝细胞癌治疗剂 商標名:MIRIPLA 批准日期:2010年1月 一般名:ミリプラチン水和物 Miriplatin Hydrate 化学名:(SP-4-2)- [(1R,2R)-Cyclohexane-1,2-diamine-N, N’]bis(tetradecanoato-O)platinum monohydrate 構造式分子式:C34H68N2O4Pt・H2O 分子量:782.01 性状:它是白色至微黄色的块状或粉末状。 它几乎不溶于水,几乎不溶于乙醇(99.5)。 处理注意事项 针对橡胶塞的针应垂直于橡胶塞面缓慢地进行。[如果倾斜摩擦,橡胶切屑可能在悬浮液中混合] 药用药理学 1.药理作用(1)细胞增殖抑制作用它显示出对人肝癌菌株HepG2,Li-7和大鼠肝癌株AH109A的细胞抑制作用。(2)抗肿瘤作用通过在肝动脉中单次施用给大鼠肝癌菌株AH109A和移植到大鼠肝脏中的人肝癌菌株Li-7显示剂量依赖性抗肿瘤作用。 2.作用机制认为毫克在体内转化为二氯-1,2-二氨基环己烷铂等,以形成与癌细胞中的DNA链共价键合的铂-DNA桥。 适应症:肝细胞癌的脂质化 用法与用量 将70mg的milliplatin悬浮在3.5mL药物溶液悬浮液中,并且每天从插入肝动脉的导管给药一次。当肿瘤血管充满悬浮液时,应完成对该药物的给药。 但是,上限设定为6mL一次(毫克为120mg)。此外,当重复给药时,应给予4周或更长的观察期。临床结果临床研究结果总结如下。 该药的延长寿命尚未得到证实。见表2临床结果表表2対象疾患名TE V注)の割合肝細胞癌26.5%(22/83)注)TE V(坏死作用100%或肿瘤减少率100%)作为肝癌治疗的直接作用判定标准 包装:70毫克:1小瓶 制造供应商:大日本住友制药公司英文版说明书
Therapeutic agent for hepatocellular carcinoma “MIRIPLA” obtainedmanufacturing and marketing approvalOctober 16, 2009Dainippon Sumitomo Pharma Co.Ltd. (Head Office: Osaka, Japan; President: Masayo Tada) announces that the Company has obtained a manufacturing and marketing approval for“MIRIPLA for intra-arterial injection 70mg” (generic name: miriplatin hydrate), a therapeutic agent for hepatocellular carcinoma in Japan as of October 16, 2009 from Ministry of Health, Labor and Welfare.“MIRIPLA” is first suspended in an oily lymphographic agent and then administered through hepatic artery into hepatocellular carcinoma.As such an oily lymphographic agent, the Company has “MIRIPLA suspension vehicle 4mL” (generic name: iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil), which is approved for suspending MIRIPLA.The manufacturing and marketing approval for this suspension vehicle was obtained on August 20, 2009 from the competent Ministry.“Lipiodolization” or “Chemo-lipiodlization” is one of the standard methods for treating hepatocellular carcinoma where an anticancer drug is suspended in an oily lymphographic agent(iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil)and then administered into hepatic artery.The Company has carried out a research program to discover an anticancer drug suitable for this treatment and succeeded in development of a lipophilic platinum complex, miriplatin which has high affinity to iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil.MIRIPLA has a high suspensibility in“MIRIPLA suspension vehicle 4mL“.Some of the characteristics of MIRIPLA are: it accumulates and stays in a tumor after the administration into hepatic artery, platinum component is released gradually over a long duration and yet exposure to entire body is minor.In clinical tests on hepatocellular carcinoma, satisfactory anti-tumor effects were confirmed not only on patients of initial treatment but also on patients who relapsed after treatment such as hepatic resection.Some side effects were observed, but they were regarded as those generally observed under chemo-lipiodolization therapy, and they were thought to be tolerable at medical institutions familiarized with this treatment. There was no adverse event on vessel disorder in hepatic artery related to this drug.The Company has an intention to launch both“MIRIPLA® for intra-arterial injection 70mg” and “MIRIPLA suspension vehicle 4mL” after they are listed on the national health insurance drug price standard.As a result of launching of MIRIPLA, the Company expects to increase the line-up of products for the liver diseases, which includes Sumiferon, a natural interferon-alpha product, and to further contribute to the total care of liver diseases.(Reference)Profile of MIRIPLA[Brand name]MIRIPLA for intra-arterial injection 70mg[Generic name]miriplatin hydrate[Content/Description]70mg of miriplatin contained in one vial(equivalent to 71.65mg of miriplatin hydrate)[Indication] Lipiodolization in hepatocellular carcinoma[Dose and Administration]70mg of miriplatin is suspended in 3.5mL of suspension vehicle for this drug, and administered once a day through catheter inserted into hepatic artery.Administration of miriplatin-suspension ends when tumor vessel is filled with the drug, provided that the upper limit should be 6mL per administration (equivalent to 120mg of miriplatin).An observation period of 4 weeks or longer is required in the case of repeated administration.[Manufacturer and Distributor]Dainippon Sumitomo PharmaProfile of“the suspension vehicle”[Brand name]MIRIPLA suspension vehicle 4mL[Generic name]iodine addition products of the ethylesters of the fatty acids obtained from poppyseed oil[Content/Description] 4mL of this liquid contained in one ampule[Indication]For suspending MIRIPLA for intra-arterial injection 70mg[Dosage and Administration] 3.5mL of this suspension vehicle added to 70mg of miriplatin[Manufacturer and Distributor]Dainippon Sumitomo Pharma.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 米铂冻干粉注射剂Miripla 70mg vial(Miriplatin Hydrate)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 米铂冻干粉注射剂Miripla 70mg vial(Miriplatin Hydrate)