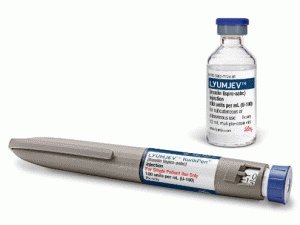
IMLYGIC 10*8(10M)PFU/ML SDV(talimogene laherparepvec)
 药店国别:
产地国家:美国
处方药:是
所属类别: 10×8(10M)/毫升/瓶
包装规格: 10×8(10M)/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:AMGEN USA, INC
原产地英文商品名:IMLYGIC 10*8(10M) PFU/ML SDV DPSH 1/EA
原产地英文药品名:TALIMOGENE LAHERPAREPVEC
中文参考商品译名:IMLYGIC悬浮液内注射 10×8(10M)/毫升/瓶
中文参考药品译名:TALIMOGENE LAHERPAREPVEC
曾用名:
简介:Imlygic(talimogen laherparepvec/T-VEC)—为第一个溶瘤病毒治疗皮肤和淋巴结黑色素瘤病变Imlygic由安进制药商(Amgen)生产的皮肤癌治疗产品,近日获美国食品药品管理局(FDA)批准上市,这也是第一款获得FDA批准的溶瘤病毒治疗产品Imlygic其实是一种病毒,存活且具有传染性,主要用于治疗不能经手术完全切除的黑色素瘤病灶。Imlygic被直接注入黑色素瘤病灶,然后在癌细胞内复制,进而导致癌细胞破裂而死。批准日期:2015年10月27日;公司:Amgen Inc.IMLYGIC(talimogene laherparepvec)悬液为病变内注射美国初次批准:2015
适应证和用途
IMLYGIC是一种遗传上修饰的溶瘤病毒治疗适用为有黑色素瘤初始手术后复发患者中不可切除的皮肤,皮下,和淋巴结病变的局部治疗。
使用限制:
IMLYGIC未曾显示改善总生存或对内脏转移有影响。
剂量和给药方法
⑴ IMLYGIC通过注射至皮肤,皮下,和/或淋巴结病变给药。
⑵ 推荐开始剂量是直至最大4mL的IMLYGIC在一个浓度106(1百万)斑块形成单位(PFU)每mL. 随后剂量应被给予直至4mL的IMLYGIC在浓度108(100百万)PFU每mL。剂型和规格注射液: 106(1百万) PFU每mL, 108 (100百万) PFU每mL在一次性小瓶中
禁忌证
⑴ 免疫力低下患者
⑵ 妊娠患者
警告和注意事项
⑴ 意外暴露于IMLYGIC:意外暴露可能导致的传播IMLYGIC和疱疹感染. 卫生保健提供者和密切接触应避免与被注射的病变,敷料,或被治疗患者的体液直接接触。是免疫力低下或妊娠卫生保健提供者不应制备或给予IMLYGIC。如发生意外暴露,被暴露隔天应清洁受影响区域。
⑵ 疱疹感染:发生发展疱疹感染患者应被忠告遵循标准卫生的习惯预防病毒传播。
⑶ 注射部位合并症:继续IMLYGIC治疗前考虑风险和获益如持久感染或延迟愈合发生发展。
⑷ 免疫介导事件:在有患有自身免疫疾病患者或发生免疫介导事件患者继续治疗前开始治疗前考虑风险和获益。
⑸ 注射部位浆细胞瘤:在有多发性骨髓瘤患者或治疗期间发生浆细胞瘤患者考虑风险和获益。
不良反应
在IMLYGIC-治疗患者最常报道的不良药物反应(≥ 25%)是疲乏,胃寒,发热,恶心,流感样疾病,和注射部位疼痛。
特殊人群中使用
⑴ 妊娠:生育潜能妇女应被忠告用IMLYGIC治疗期间使用一种避孕有效方法预防妊娠。
⑵ 哺乳:终止药物或哺乳。
包装规格
IMLYGIC 10*8(100M) PFU/ML SDV DPSH 1/EA TALIMOGENE LAHERPAREPVEC "AMGEN USA, INC." 55513-0079-01IMLYGIC 10*6(1M) PFU/ML SDV DPSH 1/EA TALIMOGENE LAHERPAREPVEC "AMGEN USA, INC." 55513-0078-01--
英文版说明书
Imlygic(talimogen laherparepvec/T-VEC)Company:AmgenApproval Status:Approved October 2015Specific Treatments:unresectable recurrent melanomaTherapeutic Areas DermatologyOncology General InformationImlygic (talimogene laherparepvec) is a genetically modified oncolytic viral therapy.Imlygic is specifically indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery.Imlygic is supplied as a suspension for intralesional injection. Imlygic should be administered by injection into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance.Imlygic is provided in single-use vials of 1 mL each in two different dose strengths: 10*6 (1 million) plaque-forming units (PFU) per mL (light green cap) – for initial dose only and 10*8 (100 million) PFU per mL (royal blue cap) – for all subsequent doses. The initial recommended dose is up to 4 mL at a concentration of 10*6 (1 million) PFU per mL. The recommended dose for subsequent administrations is up to 4 mL at a concentration of 10*8 (100 million) PFU per mL. Please see drug label for recommended dose schedule and dose volume determination.Clinical ResultsFDA ApprovalThe FDA approval of Imlygic was based on Study 005/05, referred to as OPTiM. OPTiM was a phase III, multicenter, open-label, randomized clinical trial comparing Imlygic to GM-CSF in 436 patients with advanced melanoma (Stage IIIB, IIIC, or IV) that was not surgically resectable. The subjects were randomized to receive either Imlygic (n = 295) or GM-CSF (n = 141). Imlygic was administered by intralesional injection at an initial concentration of 10*6 (1 million) PFU per mL on Day 1, followed by a concentration of 10*8 (100 million) PFU per mL on Day 21 and every 2 weeks thereafter, at a dose of up to 4 mL per visit. GM-CSF was administered subcutaneously in 28-day cycles, i.e., 125 µg/m2 daily for 14 days followed by 14 days without GM-CSF administration. The primary endpoint of the study was durable response rate (DRR), defined as the percent of patients with complete response (CR) or partial response (PR) maintained continuously for a minimum of six months. In the study, 16.3 percent of patients treated with Imlygic achieved a durable response compared to 2.1 percent of patients treated with GM-CSF (p <0.0001). Of the patients who experienced a durable response, 29.1 percent had a durable CR and 70.8 percent had a durable PR. In the study, the median time to response was 4.1 (range: 1.2 to 16.7) months in the Imlygic arm.Side EffectsAdverse effects associated with the use of Imlygic may include, but are not limited to, the following:•fatigue•chills•pyrexia•nausea•influenza-like illness•injection site painMechanism of ActionImlygic (talimogene laherparepvec) is a genetically modified oncolytic viral therapy. It was designed to replicate within tumors and to produce the immune stimulatory protein GM-CSF. Imlygic causes lysis of tumors, followed by release of tumor-derived antigens, which together with virally derived GM-CSF may promote an antitumor immune response.
药店国别:
产地国家:美国
处方药:是
所属类别: 10×8(10M)/毫升/瓶
包装规格: 10×8(10M)/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:AMGEN USA, INC
原产地英文商品名:IMLYGIC 10*8(10M) PFU/ML SDV DPSH 1/EA
原产地英文药品名:TALIMOGENE LAHERPAREPVEC
中文参考商品译名:IMLYGIC悬浮液内注射 10×8(10M)/毫升/瓶
中文参考药品译名:TALIMOGENE LAHERPAREPVEC
曾用名:
简介:Imlygic(talimogen laherparepvec/T-VEC)—为第一个溶瘤病毒治疗皮肤和淋巴结黑色素瘤病变Imlygic由安进制药商(Amgen)生产的皮肤癌治疗产品,近日获美国食品药品管理局(FDA)批准上市,这也是第一款获得FDA批准的溶瘤病毒治疗产品Imlygic其实是一种病毒,存活且具有传染性,主要用于治疗不能经手术完全切除的黑色素瘤病灶。Imlygic被直接注入黑色素瘤病灶,然后在癌细胞内复制,进而导致癌细胞破裂而死。批准日期:2015年10月27日;公司:Amgen Inc.IMLYGIC(talimogene laherparepvec)悬液为病变内注射美国初次批准:2015
适应证和用途
IMLYGIC是一种遗传上修饰的溶瘤病毒治疗适用为有黑色素瘤初始手术后复发患者中不可切除的皮肤,皮下,和淋巴结病变的局部治疗。
使用限制:
IMLYGIC未曾显示改善总生存或对内脏转移有影响。
剂量和给药方法
⑴ IMLYGIC通过注射至皮肤,皮下,和/或淋巴结病变给药。
⑵ 推荐开始剂量是直至最大4mL的IMLYGIC在一个浓度106(1百万)斑块形成单位(PFU)每mL. 随后剂量应被给予直至4mL的IMLYGIC在浓度108(100百万)PFU每mL。剂型和规格注射液: 106(1百万) PFU每mL, 108 (100百万) PFU每mL在一次性小瓶中
禁忌证
⑴ 免疫力低下患者
⑵ 妊娠患者
警告和注意事项
⑴ 意外暴露于IMLYGIC:意外暴露可能导致的传播IMLYGIC和疱疹感染. 卫生保健提供者和密切接触应避免与被注射的病变,敷料,或被治疗患者的体液直接接触。是免疫力低下或妊娠卫生保健提供者不应制备或给予IMLYGIC。如发生意外暴露,被暴露隔天应清洁受影响区域。
⑵ 疱疹感染:发生发展疱疹感染患者应被忠告遵循标准卫生的习惯预防病毒传播。
⑶ 注射部位合并症:继续IMLYGIC治疗前考虑风险和获益如持久感染或延迟愈合发生发展。
⑷ 免疫介导事件:在有患有自身免疫疾病患者或发生免疫介导事件患者继续治疗前开始治疗前考虑风险和获益。
⑸ 注射部位浆细胞瘤:在有多发性骨髓瘤患者或治疗期间发生浆细胞瘤患者考虑风险和获益。
不良反应
在IMLYGIC-治疗患者最常报道的不良药物反应(≥ 25%)是疲乏,胃寒,发热,恶心,流感样疾病,和注射部位疼痛。
特殊人群中使用
⑴ 妊娠:生育潜能妇女应被忠告用IMLYGIC治疗期间使用一种避孕有效方法预防妊娠。
⑵ 哺乳:终止药物或哺乳。
包装规格
IMLYGIC 10*8(100M) PFU/ML SDV DPSH 1/EA TALIMOGENE LAHERPAREPVEC "AMGEN USA, INC." 55513-0079-01IMLYGIC 10*6(1M) PFU/ML SDV DPSH 1/EA TALIMOGENE LAHERPAREPVEC "AMGEN USA, INC." 55513-0078-01--
英文版说明书
Imlygic(talimogen laherparepvec/T-VEC)Company:AmgenApproval Status:Approved October 2015Specific Treatments:unresectable recurrent melanomaTherapeutic Areas DermatologyOncology General InformationImlygic (talimogene laherparepvec) is a genetically modified oncolytic viral therapy.Imlygic is specifically indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery.Imlygic is supplied as a suspension for intralesional injection. Imlygic should be administered by injection into cutaneous, subcutaneous, and/or nodal lesions that are visible, palpable, or detectable by ultrasound guidance.Imlygic is provided in single-use vials of 1 mL each in two different dose strengths: 10*6 (1 million) plaque-forming units (PFU) per mL (light green cap) – for initial dose only and 10*8 (100 million) PFU per mL (royal blue cap) – for all subsequent doses. The initial recommended dose is up to 4 mL at a concentration of 10*6 (1 million) PFU per mL. The recommended dose for subsequent administrations is up to 4 mL at a concentration of 10*8 (100 million) PFU per mL. Please see drug label for recommended dose schedule and dose volume determination.Clinical ResultsFDA ApprovalThe FDA approval of Imlygic was based on Study 005/05, referred to as OPTiM. OPTiM was a phase III, multicenter, open-label, randomized clinical trial comparing Imlygic to GM-CSF in 436 patients with advanced melanoma (Stage IIIB, IIIC, or IV) that was not surgically resectable. The subjects were randomized to receive either Imlygic (n = 295) or GM-CSF (n = 141). Imlygic was administered by intralesional injection at an initial concentration of 10*6 (1 million) PFU per mL on Day 1, followed by a concentration of 10*8 (100 million) PFU per mL on Day 21 and every 2 weeks thereafter, at a dose of up to 4 mL per visit. GM-CSF was administered subcutaneously in 28-day cycles, i.e., 125 µg/m2 daily for 14 days followed by 14 days without GM-CSF administration. The primary endpoint of the study was durable response rate (DRR), defined as the percent of patients with complete response (CR) or partial response (PR) maintained continuously for a minimum of six months. In the study, 16.3 percent of patients treated with Imlygic achieved a durable response compared to 2.1 percent of patients treated with GM-CSF (p <0.0001). Of the patients who experienced a durable response, 29.1 percent had a durable CR and 70.8 percent had a durable PR. In the study, the median time to response was 4.1 (range: 1.2 to 16.7) months in the Imlygic arm.Side EffectsAdverse effects associated with the use of Imlygic may include, but are not limited to, the following:•fatigue•chills•pyrexia•nausea•influenza-like illness•injection site painMechanism of ActionImlygic (talimogene laherparepvec) is a genetically modified oncolytic viral therapy. It was designed to replicate within tumors and to produce the immune stimulatory protein GM-CSF. Imlygic causes lysis of tumors, followed by release of tumor-derived antigens, which together with virally derived GM-CSF may promote an antitumor immune response.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » IMLYGIC 10*8(10M)PFU/ML SDV(talimogene laherparepvec)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » IMLYGIC 10*8(10M)PFU/ML SDV(talimogene laherparepvec)