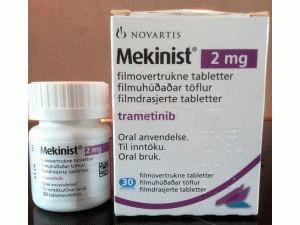
曲美替尼薄膜片Mekinist 2mg film-coated tablets
 药店国别:
产地国家:英国
处方药:是
所属类别: 2毫克/片 30片/瓶
包装规格: 2毫克/片 30片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Novartis Pharma
原产地英文商品名:Mekinist filmcoated tablets 2MG/tabs 30tabs/bottles
原产地英文药品名:TRAMETINIB
中文参考商品译名:Mekinist薄膜片 2毫克/片 30片/瓶
中文参考药品译名:曲美替尼
曾用名:
简介:部分中文曲美替尼处方资料(仅供参考)英文药:trametinib商标名:Mekinist中文名:曲美替尼片生产商:诺华制药
作用机制
Trametinib是一种有丝分裂原-活化的细胞外信号-调节的激酶1(MEK1)和MEK1和MEK2激酶活性MEK2活化的可逆性抑制剂。MEK蛋白是胞外信号-相关激酶(ERK)通路的上游调节物,它促进细胞增殖。BRAF V600E突变导致BRAF通路的结构性活化其中包括MEK1和MEK2。在体外和在体内Trametinib抑制各种BRAF V600突变-阳性肿瘤细胞的生长。Trametinib和dabrafenib靶向在RAS/RAF/MEK/ERK通路中两种不同激酶。Trametinib和dabrafenib的组合使用导致BRAF V600突变-阳性肿瘤细胞系更大的生长抑制作用在体外和与任一单独药物比较在BRAF V600突变阳性肿瘤异种移植物肿瘤生长延长抑制作用。
适应证和用途
MEKINIST是一种激酶抑制剂当被一种FDA-批准的检验检测到,适用为有BRAF V600E或V600K突变一种单药或转移黑色素瘤为有不能切除患者的治疗。MEKINIST是适用, 与dabrafenib联用,为有以下患者的治疗:
● 不能切除或转移黑色素瘤当被一种FDA-批准的检验检测到。有BRAF V600E或V600K突变.
● 转移非小细胞肺癌(NSCLC)当被一种FDA-批准的检验检测到有BRAF V600E 突变。使用的限制: MEKINIST是不适用为以前用BRAF-抑制剂治疗已进展黑色素瘤患者治疗。
剂量和给药方法
● 黑色素瘤: MEKINIST开始治疗前确证肿瘤标本存在BRAF V600E或V600K 突变。
● NSCLC: MEKINIST 与dabrafenib联用开始治疗前确证肿瘤标本存在BRAF V600E 突变。
● MEKINIST的推荐剂量方案是 2mg口服每天1次。餐前至少1小时前或餐后至少 2小时后服用MEKINIST。剂型和规格片: 0.5mg和2mg。
禁忌证
无。
警告和注意事项
● 当MEKINIST与dabrafenib被使用时可能存在新皮肤和非-皮肤原发恶性物。对新恶性病治疗开始前,当用治疗时,和治疗的终止后监视患者。
● 出血: 可能发生重大出血事件。监视出血的体征和症状。
● 结肠炎和胃肠道穿孔: 在接受MEKINIST患者中可能发生结肠炎和胃肠道穿孔。
● 静脉血栓栓塞: 在接受MEKINIST患者中可能发生深部静脉血栓形成和肺栓塞。
● 心肌病变: 治疗前,治疗的一个月后,然后其后每2至3个月评估 LVEF。
● 眼毒性: 对任何视力障碍进行眼科评价。视静脉阻塞(RVO),永久地终止MEKINIST.
● 间质性肺疾病(ILD):对新或进行性不能解释的肺症状不给MEKINIST。对治疗-相关ILD或肺炎永久地终止MEKINIST。
● 严重的发热反应:当MEKINIST是与dabrafenib使用可能发生。
● 严重的皮肤毒性: 监视对皮肤毒性和对继发性感染。终止MEKINIST对不能耐受的级别 2,或级别3或4皮疹尽管MEKINIST中断3周内不改善。
● 低血糖:在有预先存在糖尿病或低血糖患者中监视血清葡萄糖水平.
● 胚胎胎儿毒性: MEKINIST可能致胎儿危害,忠告生殖潜能女性对一个胎儿潜在风险和使用有效避孕。
不良反应
对MEKINIST作为一种单药最常见不良反应(≥20%)包括皮疹,腹泻,和淋巴水肿.对MEKINIST与dabrafenib最常见不良反应(≥20%)包括:
● 黑色素瘤: 发热,恶心,皮疹,发冷,腹泻,呕吐,高血压,和周边水肿。
● NSCLC: 发热,疲乏,恶心,呕吐,腹泻,干皮肤,食欲减退,水肿,皮疹,发冷,出血,咳嗽,和呼吸困难。
在特殊人群中使用
● 哺乳: 不要哺乳喂养。
● 生殖潜能的女性和男性: 可能损害生育力。与患者商讨对妊娠计划和预防。
曲美替尼薄膜片英文版说明书
Mekinist (Trametinib Dimethyl Sulfoxide)WARNINGS AND PRECAUTIONSReview the Full Prescribing Information for dabrafenib prior to initiation of MEKINIST in combination with dabrafenib. The following serious adverse reactions of dabrafenib as a single agent, which may occur when MEKINIST is used in combination with dabrafenib, are not described in the Full Prescribing Information for MEKINIST:• Tumor promotion in patients with BRAF wild-type melanoma• Hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiencyNew Primary MalignanciesNew primary malignancies, cutaneous and non-cutaneous, can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].Cutaneous Malignancies:In Trial 2, the incidence of basal cell carcinoma was increased in patients receiving MEKINIST in combination with dabrafenib, with an incidence of 9% (5/55) in patients receiving MEKINIST in combination with dabrafenib compared with 2% (1/53) in patients receiving dabrafenib as a single agent. The range of time to diagnosis of basal cell carcinoma was 28 to 249 days in patients receiving MEKINIST in combination with dabrafenib and was 197 days for the patient receiving dabrafenib as a single agent.Cutaneous squamous cell carcinomas (SCC), including keratoacanthoma, occurred in 7% of patients receiving MEKINIST in combination with dabrafenib and 19% of patients receiving dabrafenib as a single agent. The range of time to diagnosis of cuSCC was 136 to 197 days in the combination arm and was 9 to 197 days in the arm receiving dabrafenib as a single agent.New primary melanoma occurred in 2% (1/53) of patients receiving dabrafenib and in none of the 55 patients receiving MEKINIST in combination with dabrafenib.Perform dermatologic eva luations prior to initiation of MEKINIST in combination with dabrafenib, every 2 months while on therapy, and for up to 6 months following discontinuation of the combination. No dose modifications of MEKINIST or dabrafenib are recommended in patients who develop new primary cutaneous malignancies.Non-Cutaneous Malignancies:Based on its mechanism of action, dabrafenib may promote growth and development of malignancies with activation of RAS through mutation or other mechanisms [refer to the Full Prescribing Information for dabrafenib]. In patients receiving MEKINIST in combination with dabrafenib, four cases of non-cutaneous malignancies were identified: KRAS mutation-positive pancreatic adenocarcinoma (n = 1), recurrent NRAS mutation-positive colorectal carcinoma (n = 1), head and neck carcinoma (n = 1), and glioblastoma (n = 1). Monitor patients receiving the combination closely for signs or symptoms of non-cutaneous malignancies. If used in combination with dabrafenib, no dose modification is required for MEKINIST in patients who develop non-cutaneous malignancies. Permanently discontinue dabrafenib in patients who develop RAS mutation-positive non-cutaneous malignancies.HemorrhageHemorrhages, including major hemorrhages defined as symptomatic bleeding in a critical area or organ, can occur when MEKINIST is used in combination with dabrafenib.In Trial 2, treatment with MEKINIST in combination with dabrafenib resulted in an increased incidence and severity of any hemorrhagic event: 16% (9/55) of patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) of patients treated with dabrafenib as a single agent. The major hemorrhagic events of intracranial or gastric hemorrhage occurred in 5% (3/55) of patients treated with MEKINIST in combination with dabrafenib compared with none of the 53 patients treated with dabrafenib as a single agent. Intracranial hemorrhage was fatal in two (4%) patients receiving the combination of MEKINIST and dabrafenib.Permanently discontinue MEKINIST, and also permanently discontinue dabrafenib if administered in combination, for all Grade 4 hemorrhagic events and for any Grade 3 hemorrhagic events that do not improve. Withhold MEKINIST for up to 3 weeks for Grade 3 hemorrhagic events; if improved resume at a lower dose level. Withhold dabrafenib for Grade 3 hemorrhagic events; if improved resume at a lower dose level.Venous ThromboembolismVenous thromboembolism can occur when MEKINIST is used in combination with dabrafenib.In Trial 2, treatment with MEKINIST in combination with dabrafenib resulted in an increased incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE): 7% (4/55) of patients treated with MEKINIST in combination with dabrafenib compared with none of the 53 patients treated with dabrafenib as a single agent. Pulmonary embolism was fatal in one (2%) patient receiving the combination of MEKINIST and dabrafenib.Advise patients to immediately seek medical care if they develop symptoms of DVT or PE, such as shortness of breath, chest pain, or arm or leg swelling. Permanently discontinue MEKINIST and dabrafenib for life threatening PE. Withhold MEKINIST for uncomplicated DVT and PE for up to 3 weeks; if improved, MEKINIST may be resumed at a lower dose level. Do not modify the dose of dabrafenib [see Dosage and Administration].CardiomyopathyCardiomyopathy can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib.In Trial 1, cardiomyopathy (defined as cardiac failure, left ventricular dysfunction, or decreased left ventricular ejection fraction [LVEF]) occurred in 7% (14/211) of patients treated with MEKINIST; no chemotherapy-treated patients in Trial 1 developed cardiomyopathy. In Trial 2, cardiomyopathy occurred in 9% (5/55) of patients treated with MEKINIST in combination with dabrafenib and in none of patients treated with dabrafenib as a single agent. The median time to onset of cardiomyopathy in patients treated with MEKINIST was 63 days (range: 16 to 156 days) for Trial 1 and 86 days (range: 27 to 253 days) for Trial 2.Cardiomyopathy was identified within the first month of treatment with MEKINIST in 5 of 14 patients in Trial 1 and in 2 of 5 patients in Trial 2. Development of cardiomyopathy resulted in dose reduction (7/211) and/or discontinuation (4/211) of study drug in Trial 1, and resulted in dose reduction (4/55) and/or dose interruption (1/55) in Trial 2. Cardiomyopathy resolved in 10 of 14 (71%) patients in Trial 1 and in all 5 patients in Trial 2.Across clinical trials of MEKINIST administered either as a single agent (N = 329), or in combination with dabrafenib (N = 202), 11% and 8% of patients, respectively, developed evidence of cardiomyopathy (decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF ≥10% below baseline). Five percent and 2% in single-agent and in combination trials, respectively, demonstrated a decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF of ≥20% below baseline.Assess LVEF by echocardiogram or multigated acquisition (MUGA) scan before initiation of MEKINIST as a single agent and in combination with dabrafenib, one month after initiation, and then at 2- to 3-month intervals while on treatment. Withhold treatment with MEKINIST for up to 4 weeks if absolute LVEF value decreases by 10% from pretreatment values and is less than the lower limit of normal. For symptomatic cardiomyopathy or persistent, asymptomatic LV dysfunction that does not resolve within 4 weeks, permanently discontinue MEKINIST and withhold dabrafenib. Resume dabrafenib at the same dose upon recovery of cardiac function [see Dosage and Administration].Ocular ToxicitiesRetinal Vein Occlusion (RVO):Across all clinical trials of MEKINIST, the incidence of RVO was 0.2% (4/1,749). RVO may lead to macular edema, decreased visual function, neovascularization, and glaucoma.Urgently (within 24 hours) perform ophthalmological eva luation for patient-reported loss of vision or other visual disturbances. Permanently discontinue MEKINIST in patients with documented RVO. If MEKINIST is used in combination with dabrafenib, do not modify dabrafenib dose [see Dosage and Administration].Retinal Pigment Epithelial Detachment (RPED):Retinal pigment epithelial detachment (RPED) can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib.In Trial 1 and Trial 2, ophthalmologic examinations including retinal eva luation were performed pretreatment and at regular intervals during treatment.In Trial 1, one patient (0.5%) receiving MEKINIST developed RPED and no cases of RPED were identified in chemotherapy-treated patients. Across all clinical trials of MEKINIST, the incidence of RPED was 0.8% (14/1,749). Retinal detachments were often bilateral and multifocal, occurring in the macular region of the retina. RPED led to reduction in visual acuity that resolved after a median of 11.5 days (range: 3 to 71 days) following the interruption of dosing with MEKINIST, although Ocular Coherence Tomography (OCT) abnormalities persisted beyond a month in at least several cases.In Trial 2, one patient (2%) receiving MEKINIST in combination with dabrafenib developed RPED.Perform ophthalmological eva luation at any time a patient reports visual disturbances and compare with baseline, if available. Withhold MEKINIST if RPED is diagnosed. If resolution of the RPED is documented on repeat ophthalmological eva luation within 3 weeks, resume MEKINIST at a lower dose level. Discontinue MEKINIST if no improvement after 3 weeks. If MEKINIST is used in combination with dabrafenib, do not modify the dose of dabrafenib [see Dosage and Administration].Uveitis and Iritis:Uveitis and iritis can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].Uveitis occurred in 1% (2/202) of patients treated with MEKINIST in combination with dabrafenib.Symptomatic treatment employed in clinical trials included steroid and mydriatic ophthalmic drops. Monitor patients for visual signs and symptoms of uveitis (e.g., change in vision, photophobia, eye pain). If diagnosed, withhold dabrafenib for up to 6 weeks until uveitis/iritis resolves to Grade 0-1. If not improved, permanently discontinue dabrafenib. If MEKINIST is used in combination with dabrafenib, do not modify the dose of MEKINIST [see Dosage and Administration].Interstitial Lung DiseaseIn clinical trials of MEKINIST (N = 329) as a single agent, ILD or pneumonitis occurred in 2% of patients. In Trial 1, 2% (5/211) of patients treated with MEKINIST developed ILD or pneumonitis; all five patients required hospitalization. The median time to first presentation of ILD or pneumonitis was 160 days (range: 60 to 172 days).Withhold MEKINIST in patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Permanently discontinue MEKINIST for patients diagnosed with treatment-related ILD or pneumonitis. If MEKINIST is used in combination with dabrafenib, do not modify the dose of dabrafenib [see Dosage and Administration].Serious Febrile ReactionsSerious febrile reactions and fever of any severity accompanied by hypotension, rigors or chills, dehydration, or renal failure, can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].The incidence and severity of pyrexia are increased when MEKINIST is used in combination with dabrafenib compared with dabrafenib as a single agent [see Adverse Reactions].In Trial 2, the incidence of fever (serious and non-serious) was 71% (39/55) in patients treated with MEKINIST in combination with dabrafenib and 26% (14/53) in patients treated with dabrafenib as a single agent. Serious febrile reactions and fever of any severity accompanied by hypotension, rigors, or chills occurred in 25% (14/55) of patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) of patients treated with dabrafenib as a single agent. Fever was complicated with chills/rigors in 51% (28/55), dehydration in 9% (5/55), renal failure in 4% (2/55), and syncope in 4% (2/55) of patients in Trial 2. In patients treated with MEKINIST in combination with dabrafenib, the median time to initial onset of fever was 30 days compared with 19 days in patients treated with dabrafenib as a single agent; the median duration of fever was 6 days with the combination compared with 4 days with dabrafenib as a single agent.Across clinical trials of MEKINIST administered in combination with dabrafenib (N = 202), the incidence of pyrexia was 57% (116/202).Withhold dabrafenib for fever of 101.3ºF or higher. Withhold MEKINIST for fever higher than 104ºF. Withhold dabrafenib and MEKINIST for any serious febrile reaction or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure, and eva luate for signs and symptoms of infection. Refer to Table 2 for recommended dose modifications for adverse reactions [see Dosage and Administration]. Prophylaxis with antipyretics may be required when resuming MEKINIST or dabrafenib.Serious Skin ToxicitySerious skin toxicity can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib. Serious skin toxicity can also occur with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].In Trial 1, the overall incidence of any skin toxicity, the most common of which were rash, dermatitis acneiform rash, palmar-plantar erythrodysesthesia syndrome, and erythema, was 87% in patients treated with MEKINIST and 13% in chemotherapy-treated patients. Severe skin toxicity occurred in 12% of patients treated with MEKINIST. Skin toxicity requiring hospitalization occurred in 6% of patients treated with MEKINIST, most commonly for secondary infections of the skin requiring intravenous antibiotics or severe skin toxicity without secondary infection. In comparison, no patients treated with chemotherapy required hospitalization for severe skin toxicity or infections of the skin. The median time to onset of skin toxicity in patients treated with MEKINIST was 15 days (range: 1 to 221 days) and median time to resolution of skin toxicity was 48 days (range: 1 to 282 days). Reductions in the dose of MEKINIST were required in 12% and permanent discontinuation of MEKINIST was required in 1% of patients with skin toxicity.In Trial 2, the incidence of any skin toxicity was similar for patients receiving MEKINIST in combination with dabrafenib (65% [36/55]) compared with patients receiving dabrafenib as a single agent (68% [36/53]). The median time to onset of skin toxicity in patients treated with MEKINIST in combination with dabrafenib was 37 days (range: 1 to 225 days) and median time to resolution of skin toxicity was 33 days (range: 3 to 421 days). No patient required dose reduction or permanent discontinuation of MEKINIST or dabrafenib for skin toxicity.Across clinical trials of MEKINIST administered in combination with dabrafenib (n = 202), severe skin toxicity and secondary infection of the skin requiring hospitalization occurred in 2.5% (5/202) of patients treated with MEKINIST in combination with dabrafenib.Withhold MEKINIST, and dabrafenib if used in combination, for intolerable or severe skin toxicity. MEKINIST and dabrafenib may be resumed at lower dose levels in patients with improvement or recovery from skin toxicity within 3 weeks [see Dosage and Administration].HyperglycemiaHyperglycemia can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent. Hyperglycemia requiring an increase in the dose of, or initiation of insulin or oral hypoglycemic agent therapy occurred with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].In Trial 2, the incidence of Grade 3 hyperglycemia based on laboratory values was 5% (3/55) in patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) in patients treated with dabrafenib as a single agent.Monitor serum glucose levels as clinically appropriate during treatment with MEKINIST in combination with dabrafenib in patients with pre-existing diabetes or hyperglycemia. Advise patients to report symptoms of severe hyperglycemia.Embryofetal ToxicityBased on its mechanism of action, MEKINIST can cause fetal harm when administered to a pregnant woman. MEKINIST was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations].Advise female patients of reproductive potential to use highly effective contraception during treatment with MEKINIST and for 4 months after treatment. Advise patients to use a highly effective non-hormonal method of contraception when MEKINIST is administered in combination with dabrafenib, since dabrafenib can render hormonal contraceptives ineffective. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Use in Specific Populations (8.1, 8.6)].USE IN SPECIFIC POPULATIONSPregnancyPregnancy Category DRisk Summary: MEKINIST can cause fetal harm when administered to a pregnant woman. Trametinib was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions].Animal Data: In reproductive toxicity studies, administration of trametinib to rats during the period of organogenesis resulted in decreased fetal weights at doses greater than or equal to 0.031 mg/kg/day (approximately 0.3 times the human exposure based on AUC at the recommended dose). In rats, at a dose resulting in exposures 1.8-fold higher than the human exposure at the recommended dose, there was maternal toxicity and an increase in post-implantation loss.In pregnant rabbits, administration of trametinib during the period of organogenesis resulted in decreased fetal body weight and increased incidence of variations in ossification at doses greater than or equal to 0.039 mg/kg/day (approximately 0.08 times the human exposure at the recommended dose based on AUC). In rabbits administered trametinib at 0.15 mg/kg/day (approximately 0.3 times the human exposure at the recommended dose based on AUC) there was an increase in post-implantation loss, including total loss of pregnancy, compared with control animals.Nursing MothersIt is not known whether this drug is present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from MEKINIST, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.Pediatric UseThe safety and effectiveness of MEKINIST as a single agent or in combination with dabrafenib have not been established in pediatric patients.Adequate juvenile animal studies using trametinib have not been completed. In a repeat-dose toxicity study in juvenile rats, an increased incidence of kidney cysts and tubular deposits were noted at doses as low as 0.2 times the human exposure at the recommended adult dose of dabrafenib based on AUC. Additionally, forestomach hyperplasia, decreased bone length, and early vaginal opening were noted at doses as low as 0.8 times the human exposure at the recommended adult dose based on AUC.Geriatric UseClinical trials of MEKINIST as a single agent did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In Trial 1, 49 patients (23%) were 65 years of age and older, and 9 patients (4%) were 75 years of age and older.Across all clinical trials of MEKINIST administered in combination with dabrafenib, there was an insufficient number of patients aged 65 years and over to determine whether they respond differently from younger patients. In Trial 2, 11 patients (20%) were 65 years of age and older, and 2 patients (4%) were 75 years of age and older.Females and Males of Reproductive PotentialContraception:Females: MEKINIST can cause fetal harm when administered during pregnancy. Advise female patients of reproductive potential to use highly effective contraception during treatment and for 4 months after the last dose of MEKINIST. When MEKINIST is used in combination with dabrafenib, counsel patients to use a non-hormonal method of contraception since dabrafenib can render hormonal contraceptives ineffective. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Use in Specific Populations].Infertility:Females: MEKINIST may impair fertility in female patients [see Nonclinical Toxicology].Males: Effects on spermatogenesis have been observed in animals treated with dabrafenib. Advise male patients of the potential risk for impaired spermatogenesis, and to seek counseling on fertility and family planning options prior to starting treatment with MEKINIST in combination with dabrafenib.Hepatic ImpairmentNo formal clinical trial has been conducted to eva luate the effect of hepatic impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild hepatic impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology].The appropriate dose of MEKINIST has not been established in patients with moderate or severe hepatic impairment.Renal ImpairmentNo formal clinical trial has been conducted to eva luate the effect of renal impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild or moderate renal impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology]. The appropriate dose of MEKINIST has not been established in patients with severe renal impairment.
药店国别:
产地国家:英国
处方药:是
所属类别: 2毫克/片 30片/瓶
包装规格: 2毫克/片 30片/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Novartis Pharma
原产地英文商品名:Mekinist filmcoated tablets 2MG/tabs 30tabs/bottles
原产地英文药品名:TRAMETINIB
中文参考商品译名:Mekinist薄膜片 2毫克/片 30片/瓶
中文参考药品译名:曲美替尼
曾用名:
简介:部分中文曲美替尼处方资料(仅供参考)英文药:trametinib商标名:Mekinist中文名:曲美替尼片生产商:诺华制药
作用机制
Trametinib是一种有丝分裂原-活化的细胞外信号-调节的激酶1(MEK1)和MEK1和MEK2激酶活性MEK2活化的可逆性抑制剂。MEK蛋白是胞外信号-相关激酶(ERK)通路的上游调节物,它促进细胞增殖。BRAF V600E突变导致BRAF通路的结构性活化其中包括MEK1和MEK2。在体外和在体内Trametinib抑制各种BRAF V600突变-阳性肿瘤细胞的生长。Trametinib和dabrafenib靶向在RAS/RAF/MEK/ERK通路中两种不同激酶。Trametinib和dabrafenib的组合使用导致BRAF V600突变-阳性肿瘤细胞系更大的生长抑制作用在体外和与任一单独药物比较在BRAF V600突变阳性肿瘤异种移植物肿瘤生长延长抑制作用。
适应证和用途
MEKINIST是一种激酶抑制剂当被一种FDA-批准的检验检测到,适用为有BRAF V600E或V600K突变一种单药或转移黑色素瘤为有不能切除患者的治疗。MEKINIST是适用, 与dabrafenib联用,为有以下患者的治疗:
● 不能切除或转移黑色素瘤当被一种FDA-批准的检验检测到。有BRAF V600E或V600K突变.
● 转移非小细胞肺癌(NSCLC)当被一种FDA-批准的检验检测到有BRAF V600E 突变。使用的限制: MEKINIST是不适用为以前用BRAF-抑制剂治疗已进展黑色素瘤患者治疗。
剂量和给药方法
● 黑色素瘤: MEKINIST开始治疗前确证肿瘤标本存在BRAF V600E或V600K 突变。
● NSCLC: MEKINIST 与dabrafenib联用开始治疗前确证肿瘤标本存在BRAF V600E 突变。
● MEKINIST的推荐剂量方案是 2mg口服每天1次。餐前至少1小时前或餐后至少 2小时后服用MEKINIST。剂型和规格片: 0.5mg和2mg。
禁忌证
无。
警告和注意事项
● 当MEKINIST与dabrafenib被使用时可能存在新皮肤和非-皮肤原发恶性物。对新恶性病治疗开始前,当用治疗时,和治疗的终止后监视患者。
● 出血: 可能发生重大出血事件。监视出血的体征和症状。
● 结肠炎和胃肠道穿孔: 在接受MEKINIST患者中可能发生结肠炎和胃肠道穿孔。
● 静脉血栓栓塞: 在接受MEKINIST患者中可能发生深部静脉血栓形成和肺栓塞。
● 心肌病变: 治疗前,治疗的一个月后,然后其后每2至3个月评估 LVEF。
● 眼毒性: 对任何视力障碍进行眼科评价。视静脉阻塞(RVO),永久地终止MEKINIST.
● 间质性肺疾病(ILD):对新或进行性不能解释的肺症状不给MEKINIST。对治疗-相关ILD或肺炎永久地终止MEKINIST。
● 严重的发热反应:当MEKINIST是与dabrafenib使用可能发生。
● 严重的皮肤毒性: 监视对皮肤毒性和对继发性感染。终止MEKINIST对不能耐受的级别 2,或级别3或4皮疹尽管MEKINIST中断3周内不改善。
● 低血糖:在有预先存在糖尿病或低血糖患者中监视血清葡萄糖水平.
● 胚胎胎儿毒性: MEKINIST可能致胎儿危害,忠告生殖潜能女性对一个胎儿潜在风险和使用有效避孕。
不良反应
对MEKINIST作为一种单药最常见不良反应(≥20%)包括皮疹,腹泻,和淋巴水肿.对MEKINIST与dabrafenib最常见不良反应(≥20%)包括:
● 黑色素瘤: 发热,恶心,皮疹,发冷,腹泻,呕吐,高血压,和周边水肿。
● NSCLC: 发热,疲乏,恶心,呕吐,腹泻,干皮肤,食欲减退,水肿,皮疹,发冷,出血,咳嗽,和呼吸困难。
在特殊人群中使用
● 哺乳: 不要哺乳喂养。
● 生殖潜能的女性和男性: 可能损害生育力。与患者商讨对妊娠计划和预防。
曲美替尼薄膜片英文版说明书
Mekinist (Trametinib Dimethyl Sulfoxide)WARNINGS AND PRECAUTIONSReview the Full Prescribing Information for dabrafenib prior to initiation of MEKINIST in combination with dabrafenib. The following serious adverse reactions of dabrafenib as a single agent, which may occur when MEKINIST is used in combination with dabrafenib, are not described in the Full Prescribing Information for MEKINIST:• Tumor promotion in patients with BRAF wild-type melanoma• Hemolytic anemia in patients with glucose-6-phosphate dehydrogenase deficiencyNew Primary MalignanciesNew primary malignancies, cutaneous and non-cutaneous, can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].Cutaneous Malignancies:In Trial 2, the incidence of basal cell carcinoma was increased in patients receiving MEKINIST in combination with dabrafenib, with an incidence of 9% (5/55) in patients receiving MEKINIST in combination with dabrafenib compared with 2% (1/53) in patients receiving dabrafenib as a single agent. The range of time to diagnosis of basal cell carcinoma was 28 to 249 days in patients receiving MEKINIST in combination with dabrafenib and was 197 days for the patient receiving dabrafenib as a single agent.Cutaneous squamous cell carcinomas (SCC), including keratoacanthoma, occurred in 7% of patients receiving MEKINIST in combination with dabrafenib and 19% of patients receiving dabrafenib as a single agent. The range of time to diagnosis of cuSCC was 136 to 197 days in the combination arm and was 9 to 197 days in the arm receiving dabrafenib as a single agent.New primary melanoma occurred in 2% (1/53) of patients receiving dabrafenib and in none of the 55 patients receiving MEKINIST in combination with dabrafenib.Perform dermatologic eva luations prior to initiation of MEKINIST in combination with dabrafenib, every 2 months while on therapy, and for up to 6 months following discontinuation of the combination. No dose modifications of MEKINIST or dabrafenib are recommended in patients who develop new primary cutaneous malignancies.Non-Cutaneous Malignancies:Based on its mechanism of action, dabrafenib may promote growth and development of malignancies with activation of RAS through mutation or other mechanisms [refer to the Full Prescribing Information for dabrafenib]. In patients receiving MEKINIST in combination with dabrafenib, four cases of non-cutaneous malignancies were identified: KRAS mutation-positive pancreatic adenocarcinoma (n = 1), recurrent NRAS mutation-positive colorectal carcinoma (n = 1), head and neck carcinoma (n = 1), and glioblastoma (n = 1). Monitor patients receiving the combination closely for signs or symptoms of non-cutaneous malignancies. If used in combination with dabrafenib, no dose modification is required for MEKINIST in patients who develop non-cutaneous malignancies. Permanently discontinue dabrafenib in patients who develop RAS mutation-positive non-cutaneous malignancies.HemorrhageHemorrhages, including major hemorrhages defined as symptomatic bleeding in a critical area or organ, can occur when MEKINIST is used in combination with dabrafenib.In Trial 2, treatment with MEKINIST in combination with dabrafenib resulted in an increased incidence and severity of any hemorrhagic event: 16% (9/55) of patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) of patients treated with dabrafenib as a single agent. The major hemorrhagic events of intracranial or gastric hemorrhage occurred in 5% (3/55) of patients treated with MEKINIST in combination with dabrafenib compared with none of the 53 patients treated with dabrafenib as a single agent. Intracranial hemorrhage was fatal in two (4%) patients receiving the combination of MEKINIST and dabrafenib.Permanently discontinue MEKINIST, and also permanently discontinue dabrafenib if administered in combination, for all Grade 4 hemorrhagic events and for any Grade 3 hemorrhagic events that do not improve. Withhold MEKINIST for up to 3 weeks for Grade 3 hemorrhagic events; if improved resume at a lower dose level. Withhold dabrafenib for Grade 3 hemorrhagic events; if improved resume at a lower dose level.Venous ThromboembolismVenous thromboembolism can occur when MEKINIST is used in combination with dabrafenib.In Trial 2, treatment with MEKINIST in combination with dabrafenib resulted in an increased incidence of deep venous thrombosis (DVT) and pulmonary embolism (PE): 7% (4/55) of patients treated with MEKINIST in combination with dabrafenib compared with none of the 53 patients treated with dabrafenib as a single agent. Pulmonary embolism was fatal in one (2%) patient receiving the combination of MEKINIST and dabrafenib.Advise patients to immediately seek medical care if they develop symptoms of DVT or PE, such as shortness of breath, chest pain, or arm or leg swelling. Permanently discontinue MEKINIST and dabrafenib for life threatening PE. Withhold MEKINIST for uncomplicated DVT and PE for up to 3 weeks; if improved, MEKINIST may be resumed at a lower dose level. Do not modify the dose of dabrafenib [see Dosage and Administration].CardiomyopathyCardiomyopathy can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib.In Trial 1, cardiomyopathy (defined as cardiac failure, left ventricular dysfunction, or decreased left ventricular ejection fraction [LVEF]) occurred in 7% (14/211) of patients treated with MEKINIST; no chemotherapy-treated patients in Trial 1 developed cardiomyopathy. In Trial 2, cardiomyopathy occurred in 9% (5/55) of patients treated with MEKINIST in combination with dabrafenib and in none of patients treated with dabrafenib as a single agent. The median time to onset of cardiomyopathy in patients treated with MEKINIST was 63 days (range: 16 to 156 days) for Trial 1 and 86 days (range: 27 to 253 days) for Trial 2.Cardiomyopathy was identified within the first month of treatment with MEKINIST in 5 of 14 patients in Trial 1 and in 2 of 5 patients in Trial 2. Development of cardiomyopathy resulted in dose reduction (7/211) and/or discontinuation (4/211) of study drug in Trial 1, and resulted in dose reduction (4/55) and/or dose interruption (1/55) in Trial 2. Cardiomyopathy resolved in 10 of 14 (71%) patients in Trial 1 and in all 5 patients in Trial 2.Across clinical trials of MEKINIST administered either as a single agent (N = 329), or in combination with dabrafenib (N = 202), 11% and 8% of patients, respectively, developed evidence of cardiomyopathy (decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF ≥10% below baseline). Five percent and 2% in single-agent and in combination trials, respectively, demonstrated a decrease in LVEF below institutional lower limits of normal with an absolute decrease in LVEF of ≥20% below baseline.Assess LVEF by echocardiogram or multigated acquisition (MUGA) scan before initiation of MEKINIST as a single agent and in combination with dabrafenib, one month after initiation, and then at 2- to 3-month intervals while on treatment. Withhold treatment with MEKINIST for up to 4 weeks if absolute LVEF value decreases by 10% from pretreatment values and is less than the lower limit of normal. For symptomatic cardiomyopathy or persistent, asymptomatic LV dysfunction that does not resolve within 4 weeks, permanently discontinue MEKINIST and withhold dabrafenib. Resume dabrafenib at the same dose upon recovery of cardiac function [see Dosage and Administration].Ocular ToxicitiesRetinal Vein Occlusion (RVO):Across all clinical trials of MEKINIST, the incidence of RVO was 0.2% (4/1,749). RVO may lead to macular edema, decreased visual function, neovascularization, and glaucoma.Urgently (within 24 hours) perform ophthalmological eva luation for patient-reported loss of vision or other visual disturbances. Permanently discontinue MEKINIST in patients with documented RVO. If MEKINIST is used in combination with dabrafenib, do not modify dabrafenib dose [see Dosage and Administration].Retinal Pigment Epithelial Detachment (RPED):Retinal pigment epithelial detachment (RPED) can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib.In Trial 1 and Trial 2, ophthalmologic examinations including retinal eva luation were performed pretreatment and at regular intervals during treatment.In Trial 1, one patient (0.5%) receiving MEKINIST developed RPED and no cases of RPED were identified in chemotherapy-treated patients. Across all clinical trials of MEKINIST, the incidence of RPED was 0.8% (14/1,749). Retinal detachments were often bilateral and multifocal, occurring in the macular region of the retina. RPED led to reduction in visual acuity that resolved after a median of 11.5 days (range: 3 to 71 days) following the interruption of dosing with MEKINIST, although Ocular Coherence Tomography (OCT) abnormalities persisted beyond a month in at least several cases.In Trial 2, one patient (2%) receiving MEKINIST in combination with dabrafenib developed RPED.Perform ophthalmological eva luation at any time a patient reports visual disturbances and compare with baseline, if available. Withhold MEKINIST if RPED is diagnosed. If resolution of the RPED is documented on repeat ophthalmological eva luation within 3 weeks, resume MEKINIST at a lower dose level. Discontinue MEKINIST if no improvement after 3 weeks. If MEKINIST is used in combination with dabrafenib, do not modify the dose of dabrafenib [see Dosage and Administration].Uveitis and Iritis:Uveitis and iritis can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].Uveitis occurred in 1% (2/202) of patients treated with MEKINIST in combination with dabrafenib.Symptomatic treatment employed in clinical trials included steroid and mydriatic ophthalmic drops. Monitor patients for visual signs and symptoms of uveitis (e.g., change in vision, photophobia, eye pain). If diagnosed, withhold dabrafenib for up to 6 weeks until uveitis/iritis resolves to Grade 0-1. If not improved, permanently discontinue dabrafenib. If MEKINIST is used in combination with dabrafenib, do not modify the dose of MEKINIST [see Dosage and Administration].Interstitial Lung DiseaseIn clinical trials of MEKINIST (N = 329) as a single agent, ILD or pneumonitis occurred in 2% of patients. In Trial 1, 2% (5/211) of patients treated with MEKINIST developed ILD or pneumonitis; all five patients required hospitalization. The median time to first presentation of ILD or pneumonitis was 160 days (range: 60 to 172 days).Withhold MEKINIST in patients presenting with new or progressive pulmonary symptoms and findings including cough, dyspnea, hypoxia, pleural effusion, or infiltrates, pending clinical investigations. Permanently discontinue MEKINIST for patients diagnosed with treatment-related ILD or pneumonitis. If MEKINIST is used in combination with dabrafenib, do not modify the dose of dabrafenib [see Dosage and Administration].Serious Febrile ReactionsSerious febrile reactions and fever of any severity accompanied by hypotension, rigors or chills, dehydration, or renal failure, can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].The incidence and severity of pyrexia are increased when MEKINIST is used in combination with dabrafenib compared with dabrafenib as a single agent [see Adverse Reactions].In Trial 2, the incidence of fever (serious and non-serious) was 71% (39/55) in patients treated with MEKINIST in combination with dabrafenib and 26% (14/53) in patients treated with dabrafenib as a single agent. Serious febrile reactions and fever of any severity accompanied by hypotension, rigors, or chills occurred in 25% (14/55) of patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) of patients treated with dabrafenib as a single agent. Fever was complicated with chills/rigors in 51% (28/55), dehydration in 9% (5/55), renal failure in 4% (2/55), and syncope in 4% (2/55) of patients in Trial 2. In patients treated with MEKINIST in combination with dabrafenib, the median time to initial onset of fever was 30 days compared with 19 days in patients treated with dabrafenib as a single agent; the median duration of fever was 6 days with the combination compared with 4 days with dabrafenib as a single agent.Across clinical trials of MEKINIST administered in combination with dabrafenib (N = 202), the incidence of pyrexia was 57% (116/202).Withhold dabrafenib for fever of 101.3ºF or higher. Withhold MEKINIST for fever higher than 104ºF. Withhold dabrafenib and MEKINIST for any serious febrile reaction or fever accompanied by hypotension, rigors or chills, dehydration, or renal failure, and eva luate for signs and symptoms of infection. Refer to Table 2 for recommended dose modifications for adverse reactions [see Dosage and Administration]. Prophylaxis with antipyretics may be required when resuming MEKINIST or dabrafenib.Serious Skin ToxicitySerious skin toxicity can occur when MEKINIST is administered as a single agent or when used in combination with dabrafenib. Serious skin toxicity can also occur with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].In Trial 1, the overall incidence of any skin toxicity, the most common of which were rash, dermatitis acneiform rash, palmar-plantar erythrodysesthesia syndrome, and erythema, was 87% in patients treated with MEKINIST and 13% in chemotherapy-treated patients. Severe skin toxicity occurred in 12% of patients treated with MEKINIST. Skin toxicity requiring hospitalization occurred in 6% of patients treated with MEKINIST, most commonly for secondary infections of the skin requiring intravenous antibiotics or severe skin toxicity without secondary infection. In comparison, no patients treated with chemotherapy required hospitalization for severe skin toxicity or infections of the skin. The median time to onset of skin toxicity in patients treated with MEKINIST was 15 days (range: 1 to 221 days) and median time to resolution of skin toxicity was 48 days (range: 1 to 282 days). Reductions in the dose of MEKINIST were required in 12% and permanent discontinuation of MEKINIST was required in 1% of patients with skin toxicity.In Trial 2, the incidence of any skin toxicity was similar for patients receiving MEKINIST in combination with dabrafenib (65% [36/55]) compared with patients receiving dabrafenib as a single agent (68% [36/53]). The median time to onset of skin toxicity in patients treated with MEKINIST in combination with dabrafenib was 37 days (range: 1 to 225 days) and median time to resolution of skin toxicity was 33 days (range: 3 to 421 days). No patient required dose reduction or permanent discontinuation of MEKINIST or dabrafenib for skin toxicity.Across clinical trials of MEKINIST administered in combination with dabrafenib (n = 202), severe skin toxicity and secondary infection of the skin requiring hospitalization occurred in 2.5% (5/202) of patients treated with MEKINIST in combination with dabrafenib.Withhold MEKINIST, and dabrafenib if used in combination, for intolerable or severe skin toxicity. MEKINIST and dabrafenib may be resumed at lower dose levels in patients with improvement or recovery from skin toxicity within 3 weeks [see Dosage and Administration].HyperglycemiaHyperglycemia can occur when MEKINIST is used in combination with dabrafenib and with dabrafenib as a single agent. Hyperglycemia requiring an increase in the dose of, or initiation of insulin or oral hypoglycemic agent therapy occurred with dabrafenib as a single agent [refer to Full Prescribing Information for dabrafenib].In Trial 2, the incidence of Grade 3 hyperglycemia based on laboratory values was 5% (3/55) in patients treated with MEKINIST in combination with dabrafenib compared with 2% (1/53) in patients treated with dabrafenib as a single agent.Monitor serum glucose levels as clinically appropriate during treatment with MEKINIST in combination with dabrafenib in patients with pre-existing diabetes or hyperglycemia. Advise patients to report symptoms of severe hyperglycemia.Embryofetal ToxicityBased on its mechanism of action, MEKINIST can cause fetal harm when administered to a pregnant woman. MEKINIST was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations].Advise female patients of reproductive potential to use highly effective contraception during treatment with MEKINIST and for 4 months after treatment. Advise patients to use a highly effective non-hormonal method of contraception when MEKINIST is administered in combination with dabrafenib, since dabrafenib can render hormonal contraceptives ineffective. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Use in Specific Populations (8.1, 8.6)].USE IN SPECIFIC POPULATIONSPregnancyPregnancy Category DRisk Summary: MEKINIST can cause fetal harm when administered to a pregnant woman. Trametinib was embryotoxic and abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 0.3 times the human exposure at the recommended clinical dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Warnings and Precautions].Animal Data: In reproductive toxicity studies, administration of trametinib to rats during the period of organogenesis resulted in decreased fetal weights at doses greater than or equal to 0.031 mg/kg/day (approximately 0.3 times the human exposure based on AUC at the recommended dose). In rats, at a dose resulting in exposures 1.8-fold higher than the human exposure at the recommended dose, there was maternal toxicity and an increase in post-implantation loss.In pregnant rabbits, administration of trametinib during the period of organogenesis resulted in decreased fetal body weight and increased incidence of variations in ossification at doses greater than or equal to 0.039 mg/kg/day (approximately 0.08 times the human exposure at the recommended dose based on AUC). In rabbits administered trametinib at 0.15 mg/kg/day (approximately 0.3 times the human exposure at the recommended dose based on AUC) there was an increase in post-implantation loss, including total loss of pregnancy, compared with control animals.Nursing MothersIt is not known whether this drug is present in human milk. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from MEKINIST, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.Pediatric UseThe safety and effectiveness of MEKINIST as a single agent or in combination with dabrafenib have not been established in pediatric patients.Adequate juvenile animal studies using trametinib have not been completed. In a repeat-dose toxicity study in juvenile rats, an increased incidence of kidney cysts and tubular deposits were noted at doses as low as 0.2 times the human exposure at the recommended adult dose of dabrafenib based on AUC. Additionally, forestomach hyperplasia, decreased bone length, and early vaginal opening were noted at doses as low as 0.8 times the human exposure at the recommended adult dose based on AUC.Geriatric UseClinical trials of MEKINIST as a single agent did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In Trial 1, 49 patients (23%) were 65 years of age and older, and 9 patients (4%) were 75 years of age and older.Across all clinical trials of MEKINIST administered in combination with dabrafenib, there was an insufficient number of patients aged 65 years and over to determine whether they respond differently from younger patients. In Trial 2, 11 patients (20%) were 65 years of age and older, and 2 patients (4%) were 75 years of age and older.Females and Males of Reproductive PotentialContraception:Females: MEKINIST can cause fetal harm when administered during pregnancy. Advise female patients of reproductive potential to use highly effective contraception during treatment and for 4 months after the last dose of MEKINIST. When MEKINIST is used in combination with dabrafenib, counsel patients to use a non-hormonal method of contraception since dabrafenib can render hormonal contraceptives ineffective. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking MEKINIST [see Use in Specific Populations].Infertility:Females: MEKINIST may impair fertility in female patients [see Nonclinical Toxicology].Males: Effects on spermatogenesis have been observed in animals treated with dabrafenib. Advise male patients of the potential risk for impaired spermatogenesis, and to seek counseling on fertility and family planning options prior to starting treatment with MEKINIST in combination with dabrafenib.Hepatic ImpairmentNo formal clinical trial has been conducted to eva luate the effect of hepatic impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild hepatic impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology].The appropriate dose of MEKINIST has not been established in patients with moderate or severe hepatic impairment.Renal ImpairmentNo formal clinical trial has been conducted to eva luate the effect of renal impairment on the pharmacokinetics of trametinib. No dose adjustment is recommended in patients with mild or moderate renal impairment based on a population pharmacokinetic analysis [see Clinical Pharmacology]. The appropriate dose of MEKINIST has not been established in patients with severe renal impairment.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 曲美替尼薄膜片Mekinist 2mg film-coated tablets
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 曲美替尼薄膜片Mekinist 2mg film-coated tablets