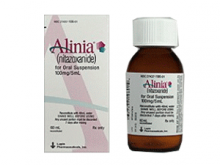
硝唑尼特口服混悬液(ALINIA ORAL SUSP 1CMG/5ML)说明书
 产地国家:美国
处方药:是
所属类别: 1毫克/5毫升 60毫升/瓶
包装规格: 1毫克/5毫升 60毫升/瓶
计价单位:瓶
生产厂家英文名:ROMARK PHARMACEUTICALS
原产地英文商品名:ALINIA ORAL SUSP 1CMG/5ML 60ML
原产地英文药品名:NITAZOXANIDE
中文参考商品译名:ALINIA口服混悬液 1毫克/5毫升 60毫升/瓶
中文参考药品译名:硝唑尼特
产地国家:美国
处方药:是
所属类别: 1毫克/5毫升 60毫升/瓶
包装规格: 1毫克/5毫升 60毫升/瓶
计价单位:瓶
生产厂家英文名:ROMARK PHARMACEUTICALS
原产地英文商品名:ALINIA ORAL SUSP 1CMG/5ML 60ML
原产地英文药品名:NITAZOXANIDE
中文参考商品译名:ALINIA口服混悬液 1毫克/5毫升 60毫升/瓶
中文参考药品译名:硝唑尼特
简介:
部分中文硝唑尼特处方资料(仅供参考) 英文名: Alinia (Nitazoxanide) 中文名: 硝唑尼特 生产商:ROMARK PHARMACEUTICALS 药品介绍: 硝唑尼特(Nitazoxanide,Alinia®, Romark Laboratories, L.C)是第一个thiazolide类药物,2002年在美国获得批准,用于治疗免疫功能健全的成人和儿童的小球隐孢子虫和蓝氏贾第虫感染。而现在大量的临床前及临床试验表明硝唑尼特对于乙肝病毒和丙肝病毒感染有活性。ALINIA(硝唑尼特 nitazoxanide)片剂,口服使用ALINIA(硝唑尼特 nitazoxanide)用于口服混悬液美国最初批准:2002年 作用机制: Nitazoxanide是一种抗原虫药[见微生物学]。 指标和用途: ALINIA是一种抗原虫药,用于治疗由贾第虫(Giardia lamblia)或隐孢子虫(Cryptosporidium parvum)引起的腹泻。 使用限制: ALINIA尚未被证明可有效治疗由HIV感染或免疫缺陷患者引起的C. parvum引起的腹泻。 剂量和给药: ALINIA片剂不应用于11岁或以下的儿科患者治疗由兰姆氏球菌或小球藻引起的腹泻的剂量:年龄剂量持续时间1至5年5 mL口服混悬液ALINIA(100 mg硝唑尼特),每12小时食用一次4至11年口服混悬液10毫升ALINIA(200毫克硝唑尼特),每12小时一次,食物3天12岁及以上每12小时一次ALINIA片剂(500毫克硝唑尼特)与食物或25毫升ALINIA口服混悬液(500毫克硝唑尼特)每12小时一次食用剂量形式和强度ALINIA片剂:500毫克ALINIA口服混悬液:100毫克/5毫升 禁忌症: 过敏症 不良反应: ≥2%的患者最常见的不良反应是腹痛,头痛,色素沉着和恶心。 药物相互作用: 当与具有窄治疗指数的其他高血浆蛋白结合药物同时施用时,可能发生对结合位点的竞争。监测不良反应。 用于特定人群儿科患者: 尚未研究ALINIA对小于1岁儿童患者口服混悬的安全性和有效性。 如何提供/存储和处理: ALINIA片剂(500毫克)ALINIA片剂是圆形,黄色,薄膜包衣片,一面是ALINIA,另一面是500片。每片含有500毫克硝唑尼特。片剂包装在12和30片的HDPE瓶中。瓶装12片NDC 67546-111-14瓶装30粒NDC 67546-111-12将片剂保存在25 oC(77 oF);短途旅行允许15 oC-30 oC(59 oF-86 oF)。[见USP受控室温]ALINIA口服混悬液(100毫克/ 5毫升)用于口服混悬液的ALINIA是一种粉红色粉末制剂,当按照指示重建时,含有100毫克硝唑尼特/5毫升。重构的悬浮液具有粉红色和草莓味。 Alinia口服混悬液有:瓶装60mL NDC 67546-212-21将未悬浮的粉末储存在25 oC(77 oF);短途旅行允许15 oC-30 oC(59 oF-86 oF)。英文版说明书:
ALINIA(nitazoxanide) Tablets, for Oral Use and Oral SuspensionRomark Pharmaceuticals announced on January 10 that it has begun to develop a new indication for Alinia (nitazoxanide) tablets in the United States - chronic hepatitis C. The company has submitted a clinical application for a new drug to the FDA and has applied for a quick approval. Clinical trials initiated in the United States will first eva luate the efficacy of nitazoxanide 500 mg twice daily for 24 weeks in patients who have previously failed combination therapy with interferon and ribavirin. Nitrozolidine is the first member of a class of compounds called thiazolides, which are small molecules that block the replication of specific viruses by inhibiting the synthesis of viral structural proteins. Studies have shown that nitazoxanide and its circulating metabolite tizoxanide can inhibit HBV and HCV in cell culture at low concentrations.A phase II, double-blind, placebo-controlled clinical trial was conducted at the Romark Digestive Disease Research Center in Egypt in early 2005. The patient was infected with HCV genotype 4, and approximately 10% of patients had previously failed treatment with peginterferon + ribavirin. This trial examined the effect of oral nitazoxanide alone on chronic hepatitis C. A phased analysis of the first 20 patients showed that 50% of patients receiving nitazoxanide (500 mg twice daily) had HCV RNA levels below the detection level at 24 weeks of treatment, compared with 0 in the placebo group. =0.03). The drug was well tolerated with no significant adverse effects and the trial is still ongoing.Alinia is already marketed in the United States, but the indications are diarrhea caused by Cryptosporidium parvum (Cp) and Giardia lamblia. Other indications under development include Clostridium difficile related diseases, Crohn's disease and chronic hepatitis C. Nitrozolidine was originally developed as a broad-spectrum anti-intestinal parasite drug and an anti-anaerobic drug, and is safer than metronidazole. It is also the first drug to be proven effective against intestinal diseases caused by Cryptosporidium parvum.Based on nitazoxanide-based antiviral activity studies, Romark began a series of Phase II clinical trials in Egypt in early 2005.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 硝唑尼特口服混悬液(ALINIA ORAL SUSP 1CMG/5ML)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 硝唑尼特口服混悬液(ALINIA ORAL SUSP 1CMG/5ML)说明书