复合索非布韦/雷迪帕韦片(Harvoni Tablets 90mg/400mg)说明书
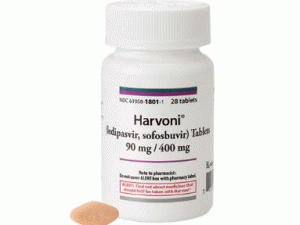 产地国家:美国
处方药:是
所属类别: (90毫克/400毫克)/片 28片/瓶
包装规格: (90毫克/400毫克)/片 28片/瓶
计价单位:瓶
生产厂家英文名:Gilead
原产地英文商品名:Harvoni(90mg/400mg)/tab 28tabs/box
原产地英文药品名:sofosbuvir/ledipasvir
中文参考商品译名:Harvoni二联复方(90毫克/400毫克)/片 28片/瓶
中文参考药品译名:索非布韦/雷迪帕韦
产地国家:美国
处方药:是
所属类别: (90毫克/400毫克)/片 28片/瓶
包装规格: (90毫克/400毫克)/片 28片/瓶
计价单位:瓶
生产厂家英文名:Gilead
原产地英文商品名:Harvoni(90mg/400mg)/tab 28tabs/box
原产地英文药品名:sofosbuvir/ledipasvir
中文参考商品译名:Harvoni二联复方(90毫克/400毫克)/片 28片/瓶
中文参考药品译名:索非布韦/雷迪帕韦
简介
近日,美国FDA批准肝病新药Harvoni(Ledipasvir/Sofosbuvir)扩展用于基因型4、5和6慢性丙型肝炎病毒(HCV)感染患者及合并感染HIV的患者。此外,Harvoni加利巴韦林12周疗程被批准作为一种替代疗法用于有24周Harvoni治疗经历的肝硬化基因型1患者。批准日期:2014年10月10日;公司:Gilead Sciences,IncHARVONI(复合索非布韦/雷迪帕韦[ledipasvir/sofosbuvir])片剂,用于口服美国最初批准:2014年警告:乙型肝炎病毒再激活的风险患有HCV和HBV的患者,查看完整的盒装警告的完整处方信息。已报道乙型肝炎病毒(HBV)再激活,在一些病例中导致暴发性肝炎,肝衰竭和死亡。最近的重大变化盒装警告02/2017适应症和用法:04/2017剂量和用量:02/2017剂量和用量:04/2017警告和注意事项:02/2017 作用机制 HARVONI是ledipasvir和sofosbuvir的固定剂量组合,它们是针对丙型肝炎病毒的直接抗病毒剂[见微生物学]。 适应症和用法 HARVONI是一种固定剂量组合的ledipasvir,一种丙型肝炎病毒(HCVNS5A抑制剂和sofosbuvir,一种HCV核苷酸类似物NS5A聚合酶抑制剂,适用于治疗慢性丙型肝炎病毒(HCV):•基因型为1,4,5或6的成人无肝硬化或伴有肝硬化的成人•基因型1感染伴失代偿性肝硬化,与利巴韦林联合治疗的成人•基因1型或4型感染的成人,肝移植受者无肝硬化或代偿性肝硬化,与利巴韦林联合治疗•12岁及以上或体重至少35kg的儿科患者,基因型1,4,5或6无肝硬化或代偿性肝硬化。 剂量和给药 开始治疗前的测试:通过测量HBsAg和抗HBc来测试所有患者的HBV感染。•推荐的成人和儿童剂量:每天一次口服或与食物一起口服一片(90毫克的阿利普沙韦和400毫克的索非布韦)。•HCV/HIV-1合并感染:适用于成人和儿童患者HCV/HIV-1合并感染,分别遵循下表中的剂量建议。•如果与利巴韦林联合使用,请遵循推荐的阿那巴韦林剂量和剂量修改。•推荐的成人治疗方案和持续时间: 成人患者人群 方案持续时间 没有肝硬化或患有复发性肝硬化的幼稚治疗(Child-PughA)HARVONI 12周 治疗 - 没有肝硬化 HARVONI 12周基因型1 经过补偿性肝硬化治疗(Child-Pugh A) HARVONI 24周 治疗初期和治疗失代偿性肝硬化(Child-Pugh B或C) HARVONI+利巴韦林12周基因型1或4治疗初期和治疗经验丰富的移植受者没有肝硬化, HARVONI+利巴韦林12周 或补偿性肝硬化(Child-Pugh A)基因型 治疗-天真和治疗 - HARVONI4,5或6 没有肝硬化或患有复发性肝硬化(Child-Pugh A) 12周•12年儿科患者的推荐治疗时间年龄和年龄或体重至少35公斤。儿科患者人群12岁及以上或体重至少35公斤 方案持续时间没有肝硬化并伴有代偿性肝硬化的幼稚治疗(Child-Pugh A)HARVONI 12周基因型1治疗 - 经历无肝硬化 HARVONI 12周经过补偿性肝硬化治疗(ChildPughA) HARVONI 24周基因型4,5或6治疗-初治和治疗经验丰富,没有肝硬化或代 HARVONI 12周 偿性肝硬化(Child-Pugh A)•对于患有严重肾功能不全或终末期肾病的患者,不能给予剂量建议。剂量形式和强度片剂:90mg的ledipasvir和400mg的sofosbuvir。禁忌症如果与利巴韦林联合使用,利巴韦林的所有禁忌症也适用于HARVONI联合治疗。警告和注意事项•乙型肝炎病毒再次激活的风险:在开始HCV治疗之前,对所有患者进行当前或之前HBV感染的证据测试。在HCV治疗和治疗后随访期间监测HCV / HBV合并感染患者的HBV再激活和肝炎突发。对临床指征的HBV感染启动适当的患者管理。•伴有胺碘酮合并症的心动过缓:服用胺碘酮的患者可能出现严重的症状性心动过缓,特别是接受β受体阻滞剂的患者,或患有潜在心脏病合并症和/或晚期肝病的患者。不建议将胺碘酮与HARVONI共同给药。对于没有其他可行治疗方案的患者,建议进行心脏监测。 不良反应 用HARVONI治疗观察到的最常见的不良反应(发生率大于或等于10%,所有等级)都是疲劳,头痛和虚弱。 药物相互作用 与胺碘酮共同给药可能导致严重的症状性心动过缓。不推荐使用HARVONI和胺碘酮。•P-gp诱导剂(例如,利福平,圣约翰草):可能改变了ledipasvir和sofosbuvir的浓度。不推荐使用HARVONI和P-gp诱导剂。•接受华法林治疗的患者建议经常监测国际标准化比值(INR)。•在用于潜在的脑膜切除术之前,请查阅完整的处方信息。 包装提供/存储和处理 HARVONI片剂为橙色,菱形,薄膜包衣,一面是“GSI”,另一面是“7985”。 每瓶含有28片(NDC 61958-1801-1),硅胶干燥剂,聚酯线圈,并用儿童防护罩封闭。在室温低于30°C(86°F)的温度下储存。•仅在原始容器中分配。•如果瓶口上的密封破损或丢失,请勿使用。英文版说明书
Ledipasvir-sofosbuvir (Harvoni)Ledipasvir is a potent inhibitor of HCV NS5A, a viral phosphoprotein that plays an important role in viral replication, assembly, and secretion. Sofosbuvir is a nucleotide analog inhibitor of hepatitis C virus NS5B polymerase—the key enzyme mediating HCV RNA replication. The triphosphate form of sofosbuvir (GS-461203) mimics the natural cellular uridine nucleotide and is incorporated by the HCV RNA polymerase into the elongating RNA primer strand, resulting in chain termination.Manufacturer for United States: The fixed-dose combination of ledipasvir and sofosbuvir (Harvoni) is manufactured by Gilead Sciences.FDA Status: On October 10, 2014, the fixed-dose combination ledipasvir-sofosbuvir (Harvoni) was approved by the FDA for the treatment of chronic hepatitis C genotype 1 infection in adults.Indications:The fixed dose combination ledipasvir-sofosbuvir (90mg/400mg) is FDA-approved for the treatment of chronic hepatitis C genotype 1 in both treatment-naive and treatment-experienced patients.The treatment duration depending on prior treatment experience and the presence or absence of cirrhosis. Treatment experience is defined as patients who have failed treatment with either peginterferon plus ribavirin or peginterferon plus ribavirin plus a HCV protease inhibitor.Genotype 1 treatment-na?ve patients with or without cirrhosis: 12 weeksGenotype 1 treatment-experienced patients without cirrhosis: 12 weeksGenotype 1 treatment-experienced patients with cirrhosis: 24 weeksTreatment experience is defined as patients who have failed treatment with either peginterferon plus ribavirin or peginterferon plus ribavirin plus a HCV protease inhibitor.Note: a treatment duration of 8 weeks can be considered in treatment-naive patients without cirrhosis who have a baseline HCV RNA level less than 6 million IU/mL.Dosing: Ledipasvir/sofosbuvir (90 mg / 400 mg) is a fixed-dose combination tablet. The recommended dosage is one tablet once daily, with or without food.Clinical Use: Sofosbuvir has pangenotypic HCV activity and ledipasvir has potent antiviral activity against genotypes 1a, 1b, 4a, 5a and 6a. The combination of ledipasvir-sofosbuvir has primarily been studied as an all-oral (interferon-free) combination regimen in treatment-naive and treatment-experienced patients with genotype 1 chronic HCV infection. The approved clinical use for ledipasvir-sofosbuvir is only for genotype 1 chronic HCV-infected adults. Ledipasvir-sofosbuvir does not have a specific indication for HIV-infected persons.Adverse Effects: Available data from clinical trials has demonstrated the combination of ledipasvir and sofosbuvir has been very well tolerated. The most common reported adverse effects are fatigue and headache.Major Drug Interactions: Ledipasvir-sofosbuvir has significant drug-drug interactions with P-gp inducers (e.g., St. John's wort and rifampin). The concomitant use of ledipasvir-sofosbuvir with P-gp inducers is not recommended. Additional drug-drug interactions may occur with ledipasvir-sofosbuvir and other medications and these are detailed in the Ledipasvir-sofosbuvir (Harvoni) Full Prescribing Information.Resistance: In vitro, ledipasvir can select for the primary NS5A mutations Q30E and Y93Y with genotype 1a and Y93H with genotype 1b. The Q30E and Y93H cause a major increase in fold resistance with ledipasvir. In a dose-ranging, 3-day monotherapy trial with ledipasvir in patients with genotype 1a or 1b infection, investigators identified a complex array of NS5A resistance associated mutations, with sites 28, 30, 31, and 93 most often involved. In phase 3 trials with sofosbuvir, investigators have observed rare treatment-emergent mutations (L159F and V321A in patients with genotype 3 infection and S282T in a patients with genotype 2). The L159F and V321A mutations did not appear to impact sofosbuvir activity, but the S282T resulted in a greater than 10-fold reduced susceptibility to sofosbuvir. Ledipasvir has excellent in vitro activity against the S282T mutants. Among patients with hepatocellular carcinoma awaiting liver transplantation who received sofosbuvir for extended treatment duration, the L159F mutation emerged in patients with genotype 1a or 2b HCV infection. Insufficient data exist regarding resistance with use of the combination ledipasvir and sofosbuvir.Prescribing Information: Ledipasvir-sofosbuvir (Harvoni) Full Prescribing Information.Summary: The fixed dose combination of ledipasvir-sofosbuvir provides a very attractive and effective one pill once a day option for treatment of genotype 1 chronic hepatitis C infection. This regimen is the first FDA-approved interferon- and ribavirin-free regimen to treat hepatitis C. Three phase 3 trials (ION-1, ION-2, and ION-3 have demonstrated SVR rates consistently above 90%. For treatment-naive, non-cirrhotic patients who have a pretreatmetn HCV RNA level less than 6 million IU/ml, the 8-week regimen will provide a major cost savings over the 12-week regimen and will actually be less expensive than a 12-week regimen of sofosbuvir plus ribavirin. The 24-week regimen for treatment-experienced cirrhotic patients is prohibitively expensive. Insufficient data exist to use ledipasvir-sofosbuvir in genotypes other than genotype 1. Although ledipasvir-sofosbuvir is not specifically FDA-approved for HIV-infected patients, it will likely generate significant interest for use in this arena.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 复合索非布韦/雷迪帕韦片(Harvoni Tablets 90mg/400mg)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 复合索非布韦/雷迪帕韦片(Harvoni Tablets 90mg/400mg)说明书