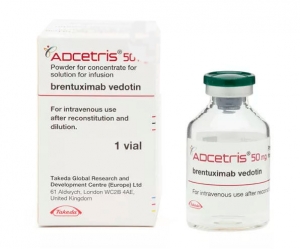
单克隆抗体ADCetris(Brentuximab Vedotin)
 药店国别:
产地国家:日本
处方药:是
所属类别: 50毫克/瓶
包装规格: 50毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Takeda
原产地英文商品名:ADCETRIS(アドセトリス 点滴静注用)50MG/VIAL
原产地英文药品名:Brentuximab Vedotin(Genetical Recombination))
中文参考商品译名:ADCETRIS(アドセトリス 点滴静注用)50毫克/瓶
中文参考药品译名:CD30单克隆抗体
药店国别:
产地国家:日本
处方药:是
所属类别: 50毫克/瓶
包装规格: 50毫克/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Takeda
原产地英文商品名:ADCETRIS(アドセトリス 点滴静注用)50MG/VIAL
原产地英文药品名:Brentuximab Vedotin(Genetical Recombination))
中文参考商品译名:ADCETRIS(アドセトリス 点滴静注用)50毫克/瓶
中文参考药品译名:CD30单克隆抗体
简介
ADCetris(Brentuximab Vedotin(Genetical Recombination))武田研发的抗体偶联药物Adcetris(brentuximab vedotin)获日本批准,用于治疗复发性或难治性CD30阳性霍奇金淋巴瘤(HL)和复发性或难治性CD30阳性系统性间变性大细胞淋巴瘤(sALCL)成人患者。ADCETRISTM (brentuximab vedotin)为静脉输注注射剂治疗类别名称抗肿瘤剂/微管抑制剂缀合的抗-CD30单克隆抗体批准上市:2014年4月 适应证和用途 ADCETRIS是一种CD30-导向抗体药物结合物适用于: (1)霍杰金淋巴瘤患者用自身干细胞移植(ASCT)失败后或不是ASCT备选者患者至少2次既往多药化疗方案失败后的治疗。 (2)有系统性间变性大细胞淋巴瘤患者至少1次既往多药化疗方案失败后的治疗。这些适应证是根据缓解率。可得到的资料没有证实用ADCETRIS报道患者结局或生存改善。剂量和给药方法(1)推荐剂量是1.8 mg/kg只作为历时30分钟静脉输注给药每3周1次。 (2)继续治疗直至最大16个疗程,疾病进展或不可接受的毒性。 剂型和规格:50mg单次使用小瓶。 禁忌证 无。 警告和注意事项 (1)周边神经病变:治疗医生应监视患者 神经病变和据此开始剂量调整。 (2)输注反应:如发生输注反应,应中断输注和开始适当医药处理。如发生过敏反应,应立即终止输注和开始适当医药处理。 (3)中性粒细胞减少:每次给ADCETRIS前监视完全血计数。如发生3或4级中性粒细胞减少,用延迟给药,减低或终止药物处理。 (4)肿瘤溶解综合征:有迅速增殖肿瘤和高肿瘤负荷患者是处在肿瘤溶解综合征风险和应严密监视这些患者和采取适当措施。 (5)Stevens-Johnson综合征:如发生Stevens-Johnson综合征,终止ADCETRIS和给予适当医药治疗。( 6)进行性多灶性白质脑病(PML):曾报道一例致命性PML。(7)妊娠中使用:可能发生胎儿危害。应忠告妊娠妇女对胎儿的潜在危害。 不良反应 最常见不良反应(≥20%)是中性粒细胞减少,周边感觉神经病变,疲乏,恶心,贫血,上呼吸道感染,腹泻,发热,皮疹,血小板减少,咳嗽,和呕吐。药物相互作用正在接受强CYP3A4抑制剂患者同时用ADCETRIS应严密监视不良反应。 特殊人群中使用 无 . 包装规格: 静脉滴注:50毫克:1瓶 生产厂商 武田药品有限公司英文版说明书
Adcetris(brentuximab vedotin) is an antibody-drug conjugate composed of a CD30-directed monoclonal antibody that is covalently linked to the antimicrotubule agent monomethyl auristatin E (MMAE).1ADCETRIS IndicationsADCETRIS(brentuximab vedotin) is indicated for treatment of patients with:Classical Hodgkin lymphoma (HL) after failure of autologous hematopoietic stem cell transplantation (auto-HSCT) or after failure of at least two prior multi-agent chemotherapy regimens in patients who are not auto-HSCT candidates.Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.Systemic anaplastic large cell lymphoma (sALCL) after failure of at least one prior multi-agent chemotherapy regimen.Accelerated approval was granted for the sALCL indication based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.Important Safety InformationBOXED WARNINGProgressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death can occur in patients receiving ADCETRIS.ContraindicationADCETRIS is contraindicated with concomitant bleomycin due to pulmonary toxicity (e.g., interstitial infiltration and/or inflammation).Warnings and PrecautionsPeripheral neuropathy: ADCETRIS treatment causes a peripheral neuropathy that is predominantly sensory. Cases of peripheral motor neuropathy have also been reported. ADCETRIS-induced peripheral neuropathy is cumulative. Monitor patients for symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain or weakness and institute dose modifications accordingly.Anaphylaxis and infusion reactions: Infusion-related reactions, including anaphylaxis, have occurred with ADCETRIS. Monitor patients during infusion. If an infusion-related reaction occurs, interrupt the infusion and institute appropriate medical management. If anaphylaxis occurs, immediately and permanently discontinue the infusion and administer appropriate medical therapy.Hematologic toxicities: Prolonged (≥1 week) severe neutropenia and Grade 3 or 4 thrombocytopenia or anemia can occur with ADCETRIS. Febrile neutropenia has been reported with ADCETRIS. Monitor complete blood counts prior to each dose of ADCETRIS and consider more frequent monitoring for patients with Grade 3 or 4 neutropenia. Monitor patients for fever. If Grade 3 or 4 neutropenia develops, consider dose delays, reductions, discontinuation, or G-CSF prophylaxis with subsequent doses.Serious infections and opportunistic infections: Infections such as pneumonia, bacteremia and sepsis or septic shock (including fatal outcomes) have been reported in patients treated with ADCETRIS. Closely monitor patients during treatment for the emergence of possible bacterial, fungal or viral infections.Tumor lysis syndrome: Closely monitor patients with rapidly proliferating tumor and high tumor burden.Increased toxicity in the presence of severe renal impairment: The frequency of ≥Grade 3 adverse reactions and deaths was greater in patients with severe renal impairment compared to patients with normal renal function. Avoid the use of ADCETRIS in patients with severe renal impairment.Increased toxicity in the presence of moderate or severe hepatic impairment: The frequency of ≥Grade 3 adverse reactions and deaths was greater in patients with moderate or severe hepatic impairment compared to pateints with normal hepatic function. Avoid the use of ADCETRIS in patients with moderate or severe hepatic impairment.Hepatotoxicity: Serious cases of hepatotoxicity, including fatal outcomes, have occurred with ADCETRIS. Cases were consistent with hepatocellular injury, including elevations of transaminases and/or bilirubin, and occurred after the first dose of ADCETRIS or rechallenge. Preexisting liver disease, elevated baseline liver enzymes, and concomitant medications may also increase the risk. Monitor liver enzymes and bilirubin. Patients experiencing new, worsening, or recurrent hepatotoxicity may require a delay, change in dose, or discontinuation of ADCETRIS.Progressive multifocal leukoencephalopathy (PML): JC virus infection resulting in PML and death has been reported in ADCETRIS-treated patients. First onset of symptoms occurred at various times from initiation of ADCETRIS therapy, with some cases occurring within 3 months of initial exposure. In addition to ADCETRIS therapy, other possible contributory factors include prior therapies and underlying disease that may cause immunosuppression. Consider the diagnosis of PML in any patient presenting with new-onset signs and symptoms of central nervous system abnormalities. Hold ADCETRIS if PML is suspected and discontinue ADCETRIS if PML is confirmed.Pulmonary Toxicity: Events of noninfectious pulmonary toxicity including pneumonitis, interstitial lung disease, and acute respiratory distress syndrome (ARDS), some with fatal outcomes, have been reported. Monitor patients for signs and symptoms of pulmonary toxicity, including cough and dyspnea. In the event of new or worsening pulmonary symptoms, hold ADCETRIS dosing during eva luation and until symptomatic improvement.Serious dermatologic reactions: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), including fatal outcomes, have been reported with ADCETRIS. If SJS or TEN occurs, discontinue ADCETRIS and administer appropriate medical therapy.Embryo-fetal toxicity: Fetal harm can occur. Advise pregnant women of the potential hazard to the fetusAdverse ReactionsADCETRIS was studied as monotherapy in 160 patients with classical HL and sALCL in two uncontrolled single-arm trials. Across both trials, the most common adverse reactions (≥20%), regardless of causality, were neutropenia, peripheral sensory neuropathy, fatigue, nausea, anemia, upper respiratory tract infection, diarrhea, pyrexia, rash, thrombocytopenia, cough and vomitingADCETRIS was studied in 329 patients with classical HL at high risk of relapse or progression post-auto-HSCT in a placebo-controlled randomized trial. The most common adverse reactions (≥20%) in the ADCETRIS-treatment arm (167 patients), regardless of causality, were neutropenia, peripheral sensory neuropathy, thrombocytopenia, anemia, upper respiratory tract infection, fatigue, peripheral motor neuropathy, nausea, cough, and diarrhea.Drug InteractionsConcomitant use of strong CYP3A4 inhibitors or inducers, or P-gp inhibitors, has the potential to affect the exposure to monomethyl auristatin E (MMAE).Use in Specific PopulationsMMAE exposure and adverse reactions are increased in patients with moderate or severe hepatic impairment or severe renal impairment. Avoid use.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 单克隆抗体ADCetris(Brentuximab Vedotin)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 单克隆抗体ADCetris(Brentuximab Vedotin)