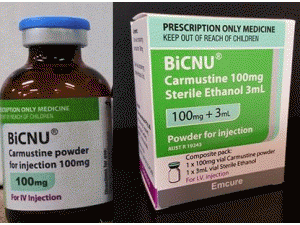
卡莫司汀冻干粉注射剂BiCNU 100mg pwd(carmustine)
 药店国别:
产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶/盒
包装规格: 100毫克/瓶 1瓶/盒
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:HERITAGE PHARMACEUTICALS INC.
原产地英文商品名:BICNU 100MG SDV PWD W/DIL 1/EA
原产地英文药品名:CARMUSTINE
中文参考商品译名:BICNU冻干粉注射剂 100毫克/瓶 1瓶/盒
中文参考药品译名:卡莫司汀
药店国别:
产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶/盒
包装规格: 100毫克/瓶 1瓶/盒
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:HERITAGE PHARMACEUTICALS INC.
原产地英文商品名:BICNU 100MG SDV PWD W/DIL 1/EA
原产地英文药品名:CARMUSTINE
中文参考商品译名:BICNU冻干粉注射剂 100毫克/瓶 1瓶/盒
中文参考药品译名:卡莫司汀
简介
BiCNU(carmustine 中文译名:卡莫司汀静脉注射)是一种用于治疗淋巴瘤,骨髓瘤和脑肿瘤化疗药物批准批准日期:2013年4月4日 公司:HERITAGE PHARMACEUTICALS-BRANDBiCNU(卡莫司汀[carmustine])注射,用于静脉注射初始美国批准:1977年 警告: 肌肉萎缩和肺部毒性请参见完整的BOXED警告的完整预定信息•抑制骨髓功能,特别是血小板减少症和白细胞减少症,是BiCNU最常见和最严重的毒性作用。监测血细胞计数。•BiCNU的肺毒性似乎与剂量有关。接受超过1400mg/m2累积剂量的患者的风险显着高于接受较少剂量的患者。 作用机制 卡莫司汀的作用机制尚不完全清楚。虽然卡莫司汀烷基化DNA和RNA,但它与其他烷化剂不具有交叉抗性。 与其他亚硝基脲一样,它可能因此通过蛋白质中氨基酸的氨基甲酰化来抑制几个关键的酶促过程。代谢物可能有助于抗肿瘤活性和卡莫司汀的毒性。 适应症和用法 BiCNU是一种亚硝基脲,在以下情况下作为单一药剂或化学治疗剂被指定为姑息治疗:脑肿瘤胶质母细胞瘤,脑干胶质瘤,成神经管细胞瘤,星形细胞瘤,室管膜瘤和转移性脑肿瘤多发性骨髓瘤与泼尼松联合使用复发或难治性霍奇金淋巴瘤与其他批准的药物联合使用复发或难治性非霍奇金淋巴瘤与其他批准的药物联合使用 剂量和用量 推荐剂量:作为单一药剂,每6周静脉注射150至200mg/m2BiCNU作为单剂量或连续2天分为每日注射75至100mg/m2。调整联合治疗剂量或骨髓储备减少的患者仅在至少2小时内缓慢静脉输注给予重构溶液。剂量形式和强度用于注射:100mg卡莫司汀冻干粉末在单剂量小瓶中用于重建,小瓶含有3mL无菌稀释液(脱水酒精注射液,USP) 禁忌症 过敏症警告和注意事项管理反应:可能发生外渗;在给药期间密切监测输注部位致癌性:可能对人类致癌。定期监测患者的搜索迹象并发现。眼部毒性:通过未经批准的动脉内颈动脉内给药途径发生。胚胎胎儿毒性:会对胎儿造成伤害。告知女性对胎儿有潜在风险并避免怀孕的生殖潜力。 不良反应 最常见的不良反应(>1%)是恶心,呕吐,肾毒性,肺炎,肺毒性,骨髓抑制。 药物相互作用 西咪替丁:伴随使用增加骨髓抑制。苯巴比妥:诱导卡莫司汀代谢,减少接触。可能导致疗效降低。苯妥英:BiCNU可降低苯妥英的功效。 用于特定人群哺乳期: 建议哺乳期妇女不要母乳喂养。 包装提供/存储和处理 提供BiCNU®(注射用卡莫司汀)。每个包装包含一个含有100毫克卡莫司汀的小瓶和一个装有3毫升无菌稀释剂的小瓶。NDC 23155-261-41存储和处理将产品和稀释剂储存在冰箱中(2°-8°C,36°-46°F)。稳定性将未开瓶的干燥药物储存在冰箱中(2°-8°C,36°-46°F)。将稀释剂小瓶存放在冰箱中(2°-8°C,36°-46°F)。未开封的BiCNU样品瓶的推荐储存可提供长达3年的稳定产品。 请勿使用PVC容器。仅从玻璃瓶或聚丙烯容器中施用BiCNU溶液。确保PVC容器不含PVC且不含DEHP。重要说明BiCNU具有低熔点(30.5°-32.0°C或86.9°-89.6°F)。药物液化并在小瓶上显示为油膜。这是分解的标志,应丢弃小瓶。如果在收到本产品时有任何充分冷藏的问题,请立即检查每个纸箱中的样品瓶。将小瓶保持在明亮的光线下进行检查。 BiCNU希望表现为极少量的干燥片状物或干燥的凝结物质。如果这很明显,BiCNU适合使用,应立即冷藏。英文版说明书
BiCNU(carmustine) for Injection, for Intravenous UseINDICATIONSBiCNU® (carmustine for injection) is indicated as palliative therapy as a single agent or in established combination therapy in the following:Brain tumors glioblastoma, brainstem glioma, medulloblastoma, astrocytoma, ependymoma, and metastatic brain tumors.Multiple myeloma in combination with prednisone.Relapsed or refractory Hodgkin's lymphoma in combination with other approved drugs.Relapsed or refractory Non-Hodgkin's lymphomas in combination with other approved drugs.DOSAGE AND ADMINISTRATIONDosageThe recommended dose of BiCNU as a single agent in previously untreated patients is 150 to 200 mg/m² intravenously every 6 weeks. Administer as a single dose or divided into daily injections such as 75 to 100 mg/m² on two successive days. Lower the dose when BiCNU is used with other myelosuppressive drugs or in patients in whom bone marrow reserve is depleted. Administer BiCNU for the duration according to the established regimen. Premedicate each dose with anti-emetics.The hematologic toxicity can be delayed and cumulative. Monitor blood counts weekly. Do not administer a repeat course of BiCNU until circulating blood elements have returned to acceptable levels (platelets above 100 Gi/L, leukocytes above 4 Gi/L and absolute neutrophil count above 1 Gi/L). The usual interval between courses is 6 weeks.eva luate renal function prior to administration and periodically during treatment. For patients with compromised renal function, monitor for toxicity more frequently. Discontinue BiCNU if the creatinine clearance is less than 10 mL/min. Do not administer BiCNU to patients with compromised renal function. Monitor transaminases and bilirubin periodically during treatment. [see ADVERSE REACTIONS].Preparation And Administration Of Intravenous SolutionDissolve BiCNU with 3 mL of the supplied sterile diluent (Dehydrated Alcohol Injection, USP).Aseptically add 27 mL Sterile Water for Injection, USP.Each mL of resulting solution contains 3.3 mg of BiCNU in 10% ethanol. Such solutions should be protected from light.The reconstituted solution is a clear, colorless to yellowish solution.Once reconstituted, the solution must be further diluted with Sodium Chloride Injection, USP or 5% Dextrose Injection, USP.Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be re-dissolved by warming the vial to room temperature with agitation.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.After reconstitution as recommended, BiCNU is stable for 24 hours under refrigeration (2°-8°C, 36°-46°F) in glass container. Examine reconstituted vials for crystal formation prior to use. If crystals are observed, they may be redissolved by warming the vial to room temperature with agitation.Vials reconstituted as directed and further diluted with 500 mL Sodium Chloride Injection, USP or 5% Dextrose Injection, USP, in glass or polypropylene containers to a concentration of 0.2 mg/mL, should be stored at room temperature, protected from light and utilized within 8 hours. These solutions are also stable 24 hours under refrigeration (2°-8°C, 36°-46°F) and an additional 6 hours at room temperature protected from light.Administer reconstituted solution by slow intravenous infusion over at least two hours. Administration of BiCNU over a period of less than two hours can lead to pain and burning at the site of injection. Monitor the injected area during the administration. The rate of administration of the intravenous infusion should not be more than 1.66 mg/m²/min.See Section 16.2 for important instructions on the storage and handling of the injection. BiCNU is a cytotoxic drug. Follow applicable special handling and disposal procedures.1The lyophilized dosage formulation contains no preservatives and is not intended for use as a multiple dose via.Accidental contact of reconstituted BiCNU with the skin has caused transient hyperpigmentation of the affected areas. Wear impervious gloves to minimize the risk of dermal exposure impervious gloves when handling vials containing BiCNU. Immediately wash the skin or mucosa thoroughly with soap and water if BiCNU lyophilized material or solution contacts the skin or mucosa1.HOW SUPPLIEDDosage Forms And StrengthsFor injection: 100 mg of carmustine as a lyophilized powder in a single-dose vial for reconstitution and a vial containing 3 mL sterile diluent (Dehydrated Alcohol Injection, USP).Storage And HandlingBiCNU® (carmustine for injection). Each package includes a vial containing 100 mg carmustine and a vial containing 3 mL sterile diluent. NDC 23155-261-41Store product and diluent in a refrigerator (2°-8°C, 36°-46°F).StabilityStore the unopened vial of the dry drug in a refrigerator (2°-8°C, 36°-46°F). Store the diluent vials in a refrigerator (2°-8°C, 36°-46°F). The recommended storage of unopened BiCNU vials provides a stable product for up to 3 years.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡莫司汀冻干粉注射剂BiCNU 100mg pwd(carmustine)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡莫司汀冻干粉注射剂BiCNU 100mg pwd(carmustine)