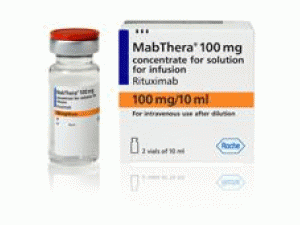
美罗华冻干粉注射剂Mabthera 100m/10ml(Rituximab)
 药店国别:
产地国家:英国
处方药:是
所属类别: 500毫克/10毫升/瓶 2瓶/盒
包装规格: 500毫克/10毫升/瓶 2瓶/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Roche
原产地英文商品名:MabThera injectabila 100mg/10ml/Vial 2Vial/box
原产地英文药品名:rituximab
中文参考商品译名:美罗华注射剂 500毫克/10毫升/瓶 2瓶/盒
中文参考药品译名:利妥昔单抗
曾用名:
简介:
部份中文美罗华处方资料(仅供参考)药品英文名Rituximab药品别名美罗华、利妥昔单抗、科妥昔单抗、MabThera、Rituxan药物剂型注射剂:500mg(50ml),100mg(10ml)。
药理作用
本品与纵贯细胞膜的CD20抗原特异性结合。该抗原位于前B细胞和成熟B淋巴细胞,但在造血干细胞、后B细胞、正常血浆细胞,或其他正常组织中不存在。该抗原表达于95%以上的B淋巴细胞型的非霍奇金淋巴瘤。在与抗体结合后,CD20不被内在化或从细胞膜上脱落。CD20不以游离抗原形式在血流中循环,因此,也就不会与抗原竞争性结合。本品与B淋巴细胞上的CD20结合,并引发B细胞溶解的免疫反应。细胞溶解的可能机制包括补体依赖性细胞毒性(CDC)和抗体依赖性细胞的细胞毒性(ADCC)。此外,体外研究表明,本品可使药物抵抗性的人体淋巴细胞对一些化疗药物的细胞毒敏感。药动学给予125mg/m2,250mg/m2或375mg/m2的本品,静脉输注,每周1次,共4次。接受本品者的血清抗体浓度随剂量的增加而上升。在给予375mg/m2的患者中,在第一次输注后,本品的血浆t1/2为68.1h,血药峰值为238.7μg/ml,平均血浆清除率为每小时0.0459L。在第4次输注后,平均血清半衰期最高浓度和血浆清除率分别为每小时189.9,480.7和0.0145L。此外,本品的血药浓度在缓解患者中的增高具有统计学意义,其典型意义是在3~6个月后仍可测到本品。在第1次给药后,中位外周B淋巴细胞数明显降低至正常水平以下,6个月后开始恢复,在治疗完成的9~12个月后恢复正常。
适应证
主要适用于中低度非霍奇金淋巴瘤,使用前需要对淋巴瘤病理切片进行CD20表达的检测,以求治疗的准确性和保证疗效。适用于复发或化疗抵抗性B淋巴细胞型的非霍奇金淋巴瘤患者。
禁忌证
1.对本品或鼠蛋白过敏者、孕妇、哺乳者禁用。
2.儿童禁用。
注意事项
1.有明显心脏病如心绞痛、心衰、哮喘、低血压等患者慎用。
2.输注速度不可过快,也不可进行静脉注射。
3.用药期间如发生变态反应或其他严重反应,应考虑减量或停药。
4.考虑本品可能引起低血压,因此,在开始使用本品时,应暂停使用抗高血压药或减量。
5.应准备好抢救过敏性休克的措施。
6.血细胞和血小板计数明显下降时,应停药。
7.用药期间,应定期检查血常规和血小板计数。
8.本品不可静脉推注。
9.避光,在2~8℃冰箱中保存。不良反应由于接受治疗的患者大多数都曾接受过多种抗癌治疗,其预后均较差,以下列出的不良反应不一定都是使用本品引起的
临床应进行细致分析
1.与输注直接相关的不良反应有发热、寒战,主要发生在第1次输注中,通常在给药后2h内发生。继而发生荨麻疹、皮疹、疲劳、头痛、瘙痒、支气管痉挛、呼吸困难、舌或喉头水肿(血管神经性水肿)、鼻炎、呕吐、一过性低血压、潮红、心律失常和肿瘤部位疼痛。
2.原有心脏病的发作,如心绞痛和充血性心衰加重。不良反应有可能随继续用药而减轻。
3.少数患者有出血倾向,常较轻且可逆。严重的血小板减少和中性粒细胞减少的发生率为1.8%。严重贫血的发生率为1.4%。
4.全身不良反应还有腹胀、腹痛、背痛、胸痛、颈痛、盗汗、汗多、皮肤干燥和输注部位疼痛。
5.心血管系统的不良反应有高血压、直立性低血压、心动过缓、心动过速、血管扩张。
6.胃肠道可见腹泻、厌食和消化不良。
7.白细胞减少、淋巴结病、高血糖、周围水肿、LDH增高、体重减轻、低血钙和血尿酸升高。
8.关节痛、肌痛、骨痛、张力过高。
9.神经系统的不良反应可见眩晕、焦虑、抑郁、感觉异常、躁动、失眠、精神紧张、嗜睡和神经炎。
10.泪腺分泌紊乱、耳痛、味觉障碍、排尿困难和血尿可能发生。
用法用量
1.作为成年患者的单一治疗药,推荐剂量为375mg/m2,静脉输注前1h先给予止痛药(对乙酰氨基酚)和抗过敏药(苯海拉明)。首剂输注速度为每小时50mg,以后每30分钟增加每小时50mg,最高可达每小时400mg。每周1次,连用4次。
2.如患者耐受,可将输入速度提高为每小时100mg,以后每30分钟增加每小时100mg,最高可达每小时400mg。药物相应作用美罗华不可与其他药物混用。关于美罗华(MabThera/Rituxan):美罗华(MabThera/Rituxan)是一种治疗性单克隆抗体,靶向结合正常和恶性B细胞表面的CD20抗原,随后调动人体天然防御,攻击和杀死标记的B细胞。骨髓内的干细胞(B细胞祖细胞)缺乏CD20抗原,从而使健康的B细胞能够在治疗后再生,并在几个月内恢复至正常水平。
英文版说明书
Mabthera infusion contains the active ingredient rituximab, which is a type of medicine known as a monoclonal antibody. It works by attacking white blood cells called B lymphocytes. This action means it can be used to treat three different diseases: cancers of the lymphatic system known as non-Hodgkins lymphomas, chronic lymphocytic leukaemia, and the inflammatory disease of the joints known as rheumatoid arthritis.What is it used for?•A type of cancer of the lymphatic system called follicular non-Hodgkins lymphoma (as a first-line treatment in combination with chemotherapy).•Follicular non-Hodgkins lymphoma that is resistant to chemotherapy or has relapsed following chemotherapy.•A type of cancer of the lymphatic system called CD20 positive diffuse large B cell non-Hodgkin's lymphoma (in combination with CHOP chemotherapy).•Chronic lymphocytic leukaemia (in combination with chemotherapy, for people who have not yet had any treatment, or whose disease has relapsed after previous treatment).•Severe active rheumatoid arthritis, in combination with another medicine called methotrexate, in adults whose arthritis has not responded well enough to treatment with disease modifiying antirheumatic drugs (DMARDs, including infliximab, etanercept or adalimumab), or who cannot take these medicines due to side effects.How does it work?Mabthera infusion contains the active ingredient rituximab, which is a type of medicine known as a monoclonal antibody. It works by attacking white blood cells called B lymphocytes. This action means it can be used to treat three different diseases: cancers of the lymphatic system known as non-Hodgkins lymphomas, chronic lymphocytic leukaemia, and the inflammatory disease of the joints known as rheumatoid arthritis.Lymphomas are cancers involving white blood cells called lymphocytes. Cancer occurs when these lymphocytes, for some reason, begin to multiply in an uncontrolled way. This can cause a lump or tumour in the lymph nodes. Lymphocytes are normally involved in fighting infection and they travel around the lymphatic system and the bloodstream to do this. If abnormal cancerous lymphocytes travel, the cancer can spread to other lymph nodes and other areas of the body. Rituximab is used to treat non-Hodgkins lymphomas that involve a sub-group of lymphocytes called B lymphocytes.RecommendedLeukaemias are also cancers involving white blood cells. In leukaemia the bone marrow produces too many immature white blood cells. These abnormal cells take up space in the bone marrow and result in less room for production of normal healthy blood cells. Rituxumab is used to treat a type of leukaemia called chronic lymphocytic leukaemia, in which too many abnormal B lymphocytes are produced.In both these conditions rituximab works by attacking the abnormal B lymphocytes.Rituximab works in a similar way to the natural antibodies produced by our immune system. Our natural antibodies recognise foreign invadors and bind to them, helping our immune systems to attack them and protect us from infections. Monoclonal antibodies like rituximab are made in laboratories. They are designed to attack particular body cells.Rituximab specifically recognises and binds to a protein called CD20, which is found on the surface of cancerous B lymphocyte cells. This triggers the immune system to attack the cancerous cells, and can also sometimes cause the cells to destroy themselves. This is how how rituximab works in treating lymphoma and leukaemia.Rituximab can also be used to treat severe forms of rheumatoid arthritis. This disease is known as an autoimmune disease, because the inflammation and damage to the joints results from overactivity in the immune system. B lymphocytes seem to play a key role in this process. Rituximab works in rheumatoid arthritis by attacking the B lymphoctyes in the same way as described above. This interrupts the process of inflammation and damage in the joints. It has been shown to slow down joint damage and improve physical functioning.How is this treatment given?•Rituximab is given by a drip into a vein (intravenous infusion). Some people can have a severe allergic reaction to rituximab, so to reduce the chance of this the first drip will usually be given over a period of a few hours. You will also usually be given some painkillers, antihistamine and possibly steroids before the drip to help prevent a reaction. If you don't have a reaction to the medicine, subsequent doses may be given over a shorter time as an outpatient.•To treat lymphoma, this medicine will either be given on its own, or in combination with other chemotherapy medicines. If used on its own, it will usually be given once a week for four weeks. If used in combination with other chemotherapy, it will be given on the same day as the other medicines, which are usually given eight times at three week intervals. Courses may be repeated. If the cancer responds to the intial treatment, maintenance treatment may be given once every two or three months for up to two years.•To treat leukaemia, this medicine will usually be given on the first day of each cycle of chemotherapy.•To treat rheumatoid arthritis, two doses of rituximab will be given, two weeks apart. This course may be repeated after six months if your doctor feels it is necessary to control your symptoms.Warning!•Molecules called cytokines may be released into the body when the B lymphocytes are destroyed by this medicine. This can lead to severe infusion-related reactions such as fever, chills, breathing difficulties, itching, blisters, throat or tongue swelling, nausea and vomiting, headache, flushing or palpitations. People whose lung function is compromised or who have cancer that has invaded the lungs, or who have a high number of cancer cells circulating in the blood or a high tumour burden are at greater risk of this syndrome. All patients must be very closely monitored, particularly those with a history of heart disease or a history of breathing problems. Your doctor may need to interrupt your infusion if you develop these symptoms. These reactions are less likely to happen after your second infusion.•You will need to have regular blood tests to check the levels of your blood cells while you are having treatment with this medicine, particularly if you are also receiving chemotherapy treatment.•This medicine can increase your risk of infections. You should tell your doctor immediately if you get any signs of infection following any of your infusions, for example, a high temperature (fever), sore throat, mouth ulcers or swollen glands.•Very rarely, some people treated with this medicine have had a serious brain infection called Progressive Multifocal Leukoencephalopathy (PML). This usually causes severe disability and can be fatal. As a result, you should tell your doctor immediately if you experience possible symptoms of this disorder, which include memory loss, trouble thinking, changes in behaviour, difficulty with walking, trouble moving one side of your body, pins and needles sensations or loss of vision.•It is not known if rituximab can affect reproductive ability, or if it can harm a developing baby when given during pregnancy. However, because it could potentially cross the placenta and attack the B cells of a developing foetus, women who could get pregnant must use an effective method of contraception to avoid pregnancy both during treatment and for 12 months after stopping treatment with this medicine.Use with caution in•People who have a high number of cancer cells circulating in the blood or a high tumour burden.•People with cancer that has invaded the lungs.•People with a history of lung disease.•People with a history of heart disease.•People who have previously been treated with chemotherapy that can have side effects on the heart.•People with low numbers of blood cells called neutrophils and platelets in their blood.•People whose immune systems have a decreased ability to fight infection and disease.•People with a history of recurrent or chronic infections.•People with a history of hepatitis B.Not to be used in•People with active severe infections.•People with a very poorly functioning immune system, for example due to previous courses of chemotherapy or radiotherapy.•People who are allergic to mouse protein.•When treating rheumatoid arthritis, this medicine should not be given to people with severe heart failure, or other severe uncontrolled heart disease.•The safety and efficacy of MabThera in children have not been established by the manufactuer. It is not recommended for children.This medicine should not be used if you are allergic to one or any of its ingredients. Please inform your doctor or pharmacist if you have previously experienced such an allergy.If you feel you have experienced an allergic reaction, stop using this medicine and inform your doctor or pharmacist immediately.Pregnancy and breastfeedingCertain medicines should not be used during pregnancy or breastfeeding. However, other medicines may be safely used in pregnancy or breastfeeding providing the benefits to the mother outweigh the risks to the unborn baby. Always inform your doctor if you are pregnant or planning a pregnancy, before using any medicine.•It is not known if rituximab can affect reproductive ability, or if it can harm a foetus when given during pregnancy. However, because it could potentially cross the placenta and attack the B cells of a developing baby, it should not be given to pregnant women unless the potential benefit outweighs the possible risk.•Women who could get pregnant must use an effective method of contraception to prevent pregnancy, both during treatment with this medicine and for 12 months after their last drip. Seek further medical advice from your doctor.•It is not known if this medicine passes into breast milk. However, because it may pass into breast milk and harm a nursing infant, it should not be given to women who are breastfeeding. Women who have been treated with Mabthera should not breastfeed for 12 months after their last drip. Seek medical advice from your doctor.Side effectsMedicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that are known to be associated with this medicine. Just because a side effect is stated here does not mean that all people using this medicine will experience that or any side effect. Side effects are less likely if you are being treated with this medicine for rheumatoid arthritis.•Fever (pyrexia) or chills.•Weakness or loss of strength (asthenia).•Headache.•Decreased numer of white blood cells, platelets or red blood cells in the blood.•Increased susceptibility to infections - see warning section above.•Rash or itching.•Hair loss.•Sweating.•Disturbances of the gut such as nausea and vomiting, diarrhoea, constipation, abdominal pain or swelling, indigestion.•Runny or itchy nose.•Severe swelling of lips, face or tongue (angioedema).•Decrease or increase in blood pressure.•Dizziness.•Flushing.•Pins and needles or numb sensations.•Difficulty sleeping (insomnia).•Anxiety, agitation, nervousness or depression.•Sensation of ringing or other noise in the ears (tinnitus).•Breathing difficulties due to a narrowing of the airways (bronchospasm) or other lung problems.•Throat irritation.•Pain in the muscles, joints, back or neck.•Increased blood sugar level (hyperglycaemia).•Swollen ankles due to fluid retention.•Tumour pain.•Heart problems, such as abnormal heart beats (arrhythmias), heart failure, chest pain or heart attack.The side effects listed above may not include all of the side effects reported by the medicine's manufacturer.For more information about any other possible risks associated with this medicine, please read the information provided with the medicine or consult your doctor or pharmacist.How can this medicine affect other medicines?Always tell your doctor if you are taking any other medicines, including over-the-counter and herbal medicines, before starting treatment with rituximab. Similarly, always seek advice from your doctor or pharmacist before taking any new medicines while you are receiving treatment with rituximab, so they can check that the combination is safe.Rituximab may cause low blood pressure and enhance the effect of blood pressure lowering medicines. For this reason, if you are taking medicines for high blood pressure your doctor may want you to stop taking them 12 hours before your Mabthera drip.If you are having this medicine for rheumatoid arthritis, you should tell your doctor if you are likely to need any vaccinations, for example travel vaccinations, before you have your drip. As this medicine attacks your B cells, which are part of your immune system, it can make it hard for your body to produce antibodies. This means that vaccines may potentially be less effective if given during treatment, and live vaccines may cause serious infections. For this reason, if you need to have any vaccinations, these should be completed at least four weeks before your first MabThera drip. Live vaccines are not recommended while your B cells are depleted (for a few months after your drip). Live vaccines include measles, mumps, rubella, MMR, oral polio, oral typhoid and yellow fever.The use of this medicine in combination with other medicines for rheumatoid arthritis has not been studied (with the exception of methotrexate). If you are prescribed any other disease modifiying antirheumatic drugs (DMARDs, eg infliximab, etanercept, adalimumab, gold, penicillamine, chloroquine, sulfasalazine) after your Mabthera drip, you should let your doctor know if you experience any side effects, particularly any signs of infection such as fever.Other medicines containing the same active ingredientThere are currently no other medicines available in the UK that contain rituximab as the active ingredient.
药店国别:
产地国家:英国
处方药:是
所属类别: 500毫克/10毫升/瓶 2瓶/盒
包装规格: 500毫克/10毫升/瓶 2瓶/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Roche
原产地英文商品名:MabThera injectabila 100mg/10ml/Vial 2Vial/box
原产地英文药品名:rituximab
中文参考商品译名:美罗华注射剂 500毫克/10毫升/瓶 2瓶/盒
中文参考药品译名:利妥昔单抗
曾用名:
简介:
部份中文美罗华处方资料(仅供参考)药品英文名Rituximab药品别名美罗华、利妥昔单抗、科妥昔单抗、MabThera、Rituxan药物剂型注射剂:500mg(50ml),100mg(10ml)。
药理作用
本品与纵贯细胞膜的CD20抗原特异性结合。该抗原位于前B细胞和成熟B淋巴细胞,但在造血干细胞、后B细胞、正常血浆细胞,或其他正常组织中不存在。该抗原表达于95%以上的B淋巴细胞型的非霍奇金淋巴瘤。在与抗体结合后,CD20不被内在化或从细胞膜上脱落。CD20不以游离抗原形式在血流中循环,因此,也就不会与抗原竞争性结合。本品与B淋巴细胞上的CD20结合,并引发B细胞溶解的免疫反应。细胞溶解的可能机制包括补体依赖性细胞毒性(CDC)和抗体依赖性细胞的细胞毒性(ADCC)。此外,体外研究表明,本品可使药物抵抗性的人体淋巴细胞对一些化疗药物的细胞毒敏感。药动学给予125mg/m2,250mg/m2或375mg/m2的本品,静脉输注,每周1次,共4次。接受本品者的血清抗体浓度随剂量的增加而上升。在给予375mg/m2的患者中,在第一次输注后,本品的血浆t1/2为68.1h,血药峰值为238.7μg/ml,平均血浆清除率为每小时0.0459L。在第4次输注后,平均血清半衰期最高浓度和血浆清除率分别为每小时189.9,480.7和0.0145L。此外,本品的血药浓度在缓解患者中的增高具有统计学意义,其典型意义是在3~6个月后仍可测到本品。在第1次给药后,中位外周B淋巴细胞数明显降低至正常水平以下,6个月后开始恢复,在治疗完成的9~12个月后恢复正常。
适应证
主要适用于中低度非霍奇金淋巴瘤,使用前需要对淋巴瘤病理切片进行CD20表达的检测,以求治疗的准确性和保证疗效。适用于复发或化疗抵抗性B淋巴细胞型的非霍奇金淋巴瘤患者。
禁忌证
1.对本品或鼠蛋白过敏者、孕妇、哺乳者禁用。
2.儿童禁用。
注意事项
1.有明显心脏病如心绞痛、心衰、哮喘、低血压等患者慎用。
2.输注速度不可过快,也不可进行静脉注射。
3.用药期间如发生变态反应或其他严重反应,应考虑减量或停药。
4.考虑本品可能引起低血压,因此,在开始使用本品时,应暂停使用抗高血压药或减量。
5.应准备好抢救过敏性休克的措施。
6.血细胞和血小板计数明显下降时,应停药。
7.用药期间,应定期检查血常规和血小板计数。
8.本品不可静脉推注。
9.避光,在2~8℃冰箱中保存。不良反应由于接受治疗的患者大多数都曾接受过多种抗癌治疗,其预后均较差,以下列出的不良反应不一定都是使用本品引起的
临床应进行细致分析
1.与输注直接相关的不良反应有发热、寒战,主要发生在第1次输注中,通常在给药后2h内发生。继而发生荨麻疹、皮疹、疲劳、头痛、瘙痒、支气管痉挛、呼吸困难、舌或喉头水肿(血管神经性水肿)、鼻炎、呕吐、一过性低血压、潮红、心律失常和肿瘤部位疼痛。
2.原有心脏病的发作,如心绞痛和充血性心衰加重。不良反应有可能随继续用药而减轻。
3.少数患者有出血倾向,常较轻且可逆。严重的血小板减少和中性粒细胞减少的发生率为1.8%。严重贫血的发生率为1.4%。
4.全身不良反应还有腹胀、腹痛、背痛、胸痛、颈痛、盗汗、汗多、皮肤干燥和输注部位疼痛。
5.心血管系统的不良反应有高血压、直立性低血压、心动过缓、心动过速、血管扩张。
6.胃肠道可见腹泻、厌食和消化不良。
7.白细胞减少、淋巴结病、高血糖、周围水肿、LDH增高、体重减轻、低血钙和血尿酸升高。
8.关节痛、肌痛、骨痛、张力过高。
9.神经系统的不良反应可见眩晕、焦虑、抑郁、感觉异常、躁动、失眠、精神紧张、嗜睡和神经炎。
10.泪腺分泌紊乱、耳痛、味觉障碍、排尿困难和血尿可能发生。
用法用量
1.作为成年患者的单一治疗药,推荐剂量为375mg/m2,静脉输注前1h先给予止痛药(对乙酰氨基酚)和抗过敏药(苯海拉明)。首剂输注速度为每小时50mg,以后每30分钟增加每小时50mg,最高可达每小时400mg。每周1次,连用4次。
2.如患者耐受,可将输入速度提高为每小时100mg,以后每30分钟增加每小时100mg,最高可达每小时400mg。药物相应作用美罗华不可与其他药物混用。关于美罗华(MabThera/Rituxan):美罗华(MabThera/Rituxan)是一种治疗性单克隆抗体,靶向结合正常和恶性B细胞表面的CD20抗原,随后调动人体天然防御,攻击和杀死标记的B细胞。骨髓内的干细胞(B细胞祖细胞)缺乏CD20抗原,从而使健康的B细胞能够在治疗后再生,并在几个月内恢复至正常水平。
英文版说明书
Mabthera infusion contains the active ingredient rituximab, which is a type of medicine known as a monoclonal antibody. It works by attacking white blood cells called B lymphocytes. This action means it can be used to treat three different diseases: cancers of the lymphatic system known as non-Hodgkins lymphomas, chronic lymphocytic leukaemia, and the inflammatory disease of the joints known as rheumatoid arthritis.What is it used for?•A type of cancer of the lymphatic system called follicular non-Hodgkins lymphoma (as a first-line treatment in combination with chemotherapy).•Follicular non-Hodgkins lymphoma that is resistant to chemotherapy or has relapsed following chemotherapy.•A type of cancer of the lymphatic system called CD20 positive diffuse large B cell non-Hodgkin's lymphoma (in combination with CHOP chemotherapy).•Chronic lymphocytic leukaemia (in combination with chemotherapy, for people who have not yet had any treatment, or whose disease has relapsed after previous treatment).•Severe active rheumatoid arthritis, in combination with another medicine called methotrexate, in adults whose arthritis has not responded well enough to treatment with disease modifiying antirheumatic drugs (DMARDs, including infliximab, etanercept or adalimumab), or who cannot take these medicines due to side effects.How does it work?Mabthera infusion contains the active ingredient rituximab, which is a type of medicine known as a monoclonal antibody. It works by attacking white blood cells called B lymphocytes. This action means it can be used to treat three different diseases: cancers of the lymphatic system known as non-Hodgkins lymphomas, chronic lymphocytic leukaemia, and the inflammatory disease of the joints known as rheumatoid arthritis.Lymphomas are cancers involving white blood cells called lymphocytes. Cancer occurs when these lymphocytes, for some reason, begin to multiply in an uncontrolled way. This can cause a lump or tumour in the lymph nodes. Lymphocytes are normally involved in fighting infection and they travel around the lymphatic system and the bloodstream to do this. If abnormal cancerous lymphocytes travel, the cancer can spread to other lymph nodes and other areas of the body. Rituximab is used to treat non-Hodgkins lymphomas that involve a sub-group of lymphocytes called B lymphocytes.RecommendedLeukaemias are also cancers involving white blood cells. In leukaemia the bone marrow produces too many immature white blood cells. These abnormal cells take up space in the bone marrow and result in less room for production of normal healthy blood cells. Rituxumab is used to treat a type of leukaemia called chronic lymphocytic leukaemia, in which too many abnormal B lymphocytes are produced.In both these conditions rituximab works by attacking the abnormal B lymphocytes.Rituximab works in a similar way to the natural antibodies produced by our immune system. Our natural antibodies recognise foreign invadors and bind to them, helping our immune systems to attack them and protect us from infections. Monoclonal antibodies like rituximab are made in laboratories. They are designed to attack particular body cells.Rituximab specifically recognises and binds to a protein called CD20, which is found on the surface of cancerous B lymphocyte cells. This triggers the immune system to attack the cancerous cells, and can also sometimes cause the cells to destroy themselves. This is how how rituximab works in treating lymphoma and leukaemia.Rituximab can also be used to treat severe forms of rheumatoid arthritis. This disease is known as an autoimmune disease, because the inflammation and damage to the joints results from overactivity in the immune system. B lymphocytes seem to play a key role in this process. Rituximab works in rheumatoid arthritis by attacking the B lymphoctyes in the same way as described above. This interrupts the process of inflammation and damage in the joints. It has been shown to slow down joint damage and improve physical functioning.How is this treatment given?•Rituximab is given by a drip into a vein (intravenous infusion). Some people can have a severe allergic reaction to rituximab, so to reduce the chance of this the first drip will usually be given over a period of a few hours. You will also usually be given some painkillers, antihistamine and possibly steroids before the drip to help prevent a reaction. If you don't have a reaction to the medicine, subsequent doses may be given over a shorter time as an outpatient.•To treat lymphoma, this medicine will either be given on its own, or in combination with other chemotherapy medicines. If used on its own, it will usually be given once a week for four weeks. If used in combination with other chemotherapy, it will be given on the same day as the other medicines, which are usually given eight times at three week intervals. Courses may be repeated. If the cancer responds to the intial treatment, maintenance treatment may be given once every two or three months for up to two years.•To treat leukaemia, this medicine will usually be given on the first day of each cycle of chemotherapy.•To treat rheumatoid arthritis, two doses of rituximab will be given, two weeks apart. This course may be repeated after six months if your doctor feels it is necessary to control your symptoms.Warning!•Molecules called cytokines may be released into the body when the B lymphocytes are destroyed by this medicine. This can lead to severe infusion-related reactions such as fever, chills, breathing difficulties, itching, blisters, throat or tongue swelling, nausea and vomiting, headache, flushing or palpitations. People whose lung function is compromised or who have cancer that has invaded the lungs, or who have a high number of cancer cells circulating in the blood or a high tumour burden are at greater risk of this syndrome. All patients must be very closely monitored, particularly those with a history of heart disease or a history of breathing problems. Your doctor may need to interrupt your infusion if you develop these symptoms. These reactions are less likely to happen after your second infusion.•You will need to have regular blood tests to check the levels of your blood cells while you are having treatment with this medicine, particularly if you are also receiving chemotherapy treatment.•This medicine can increase your risk of infections. You should tell your doctor immediately if you get any signs of infection following any of your infusions, for example, a high temperature (fever), sore throat, mouth ulcers or swollen glands.•Very rarely, some people treated with this medicine have had a serious brain infection called Progressive Multifocal Leukoencephalopathy (PML). This usually causes severe disability and can be fatal. As a result, you should tell your doctor immediately if you experience possible symptoms of this disorder, which include memory loss, trouble thinking, changes in behaviour, difficulty with walking, trouble moving one side of your body, pins and needles sensations or loss of vision.•It is not known if rituximab can affect reproductive ability, or if it can harm a developing baby when given during pregnancy. However, because it could potentially cross the placenta and attack the B cells of a developing foetus, women who could get pregnant must use an effective method of contraception to avoid pregnancy both during treatment and for 12 months after stopping treatment with this medicine.Use with caution in•People who have a high number of cancer cells circulating in the blood or a high tumour burden.•People with cancer that has invaded the lungs.•People with a history of lung disease.•People with a history of heart disease.•People who have previously been treated with chemotherapy that can have side effects on the heart.•People with low numbers of blood cells called neutrophils and platelets in their blood.•People whose immune systems have a decreased ability to fight infection and disease.•People with a history of recurrent or chronic infections.•People with a history of hepatitis B.Not to be used in•People with active severe infections.•People with a very poorly functioning immune system, for example due to previous courses of chemotherapy or radiotherapy.•People who are allergic to mouse protein.•When treating rheumatoid arthritis, this medicine should not be given to people with severe heart failure, or other severe uncontrolled heart disease.•The safety and efficacy of MabThera in children have not been established by the manufactuer. It is not recommended for children.This medicine should not be used if you are allergic to one or any of its ingredients. Please inform your doctor or pharmacist if you have previously experienced such an allergy.If you feel you have experienced an allergic reaction, stop using this medicine and inform your doctor or pharmacist immediately.Pregnancy and breastfeedingCertain medicines should not be used during pregnancy or breastfeeding. However, other medicines may be safely used in pregnancy or breastfeeding providing the benefits to the mother outweigh the risks to the unborn baby. Always inform your doctor if you are pregnant or planning a pregnancy, before using any medicine.•It is not known if rituximab can affect reproductive ability, or if it can harm a foetus when given during pregnancy. However, because it could potentially cross the placenta and attack the B cells of a developing baby, it should not be given to pregnant women unless the potential benefit outweighs the possible risk.•Women who could get pregnant must use an effective method of contraception to prevent pregnancy, both during treatment with this medicine and for 12 months after their last drip. Seek further medical advice from your doctor.•It is not known if this medicine passes into breast milk. However, because it may pass into breast milk and harm a nursing infant, it should not be given to women who are breastfeeding. Women who have been treated with Mabthera should not breastfeed for 12 months after their last drip. Seek medical advice from your doctor.Side effectsMedicines and their possible side effects can affect individual people in different ways. The following are some of the side effects that are known to be associated with this medicine. Just because a side effect is stated here does not mean that all people using this medicine will experience that or any side effect. Side effects are less likely if you are being treated with this medicine for rheumatoid arthritis.•Fever (pyrexia) or chills.•Weakness or loss of strength (asthenia).•Headache.•Decreased numer of white blood cells, platelets or red blood cells in the blood.•Increased susceptibility to infections - see warning section above.•Rash or itching.•Hair loss.•Sweating.•Disturbances of the gut such as nausea and vomiting, diarrhoea, constipation, abdominal pain or swelling, indigestion.•Runny or itchy nose.•Severe swelling of lips, face or tongue (angioedema).•Decrease or increase in blood pressure.•Dizziness.•Flushing.•Pins and needles or numb sensations.•Difficulty sleeping (insomnia).•Anxiety, agitation, nervousness or depression.•Sensation of ringing or other noise in the ears (tinnitus).•Breathing difficulties due to a narrowing of the airways (bronchospasm) or other lung problems.•Throat irritation.•Pain in the muscles, joints, back or neck.•Increased blood sugar level (hyperglycaemia).•Swollen ankles due to fluid retention.•Tumour pain.•Heart problems, such as abnormal heart beats (arrhythmias), heart failure, chest pain or heart attack.The side effects listed above may not include all of the side effects reported by the medicine's manufacturer.For more information about any other possible risks associated with this medicine, please read the information provided with the medicine or consult your doctor or pharmacist.How can this medicine affect other medicines?Always tell your doctor if you are taking any other medicines, including over-the-counter and herbal medicines, before starting treatment with rituximab. Similarly, always seek advice from your doctor or pharmacist before taking any new medicines while you are receiving treatment with rituximab, so they can check that the combination is safe.Rituximab may cause low blood pressure and enhance the effect of blood pressure lowering medicines. For this reason, if you are taking medicines for high blood pressure your doctor may want you to stop taking them 12 hours before your Mabthera drip.If you are having this medicine for rheumatoid arthritis, you should tell your doctor if you are likely to need any vaccinations, for example travel vaccinations, before you have your drip. As this medicine attacks your B cells, which are part of your immune system, it can make it hard for your body to produce antibodies. This means that vaccines may potentially be less effective if given during treatment, and live vaccines may cause serious infections. For this reason, if you need to have any vaccinations, these should be completed at least four weeks before your first MabThera drip. Live vaccines are not recommended while your B cells are depleted (for a few months after your drip). Live vaccines include measles, mumps, rubella, MMR, oral polio, oral typhoid and yellow fever.The use of this medicine in combination with other medicines for rheumatoid arthritis has not been studied (with the exception of methotrexate). If you are prescribed any other disease modifiying antirheumatic drugs (DMARDs, eg infliximab, etanercept, adalimumab, gold, penicillamine, chloroquine, sulfasalazine) after your Mabthera drip, you should let your doctor know if you experience any side effects, particularly any signs of infection such as fever.Other medicines containing the same active ingredientThere are currently no other medicines available in the UK that contain rituximab as the active ingredient.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 美罗华冻干粉注射剂Mabthera 100m/10ml(Rituximab)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 美罗华冻干粉注射剂Mabthera 100m/10ml(Rituximab)