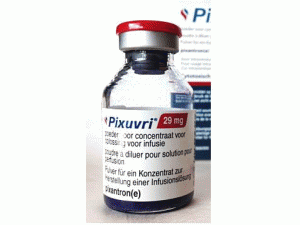
匹杉琼冻干粉注射剂Pixuvri(pixantrone dimaleate)
 药店国别:
产地国家:瑞士
处方药:是
所属类别: 29毫克/毫升/瓶
包装规格: 29毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Cell Therapeutics
原产地英文商品名:Pixuvri 29MG/ML
原产地英文药品名:pixantrone dimaleate
中文参考商品译名:Pixuvri冻干粉注射剂 29毫克/毫升/瓶
中文参考药品译名:马来酸匹杉琼
曾用名:
简介:
马来酸匹杉琼冻干粉注射剂Pixuvri(pixantrone dimaleate)马来酸匹杉琼(pixantrone dimaleate)是一种细胞毒性氮杂蒽二酮。和已经批准的蒽环类抗生素(多柔比星及其他)以及蒽二酮(米托蒽醌)不同,匹杉琼是一种弱拓扑异构酶II抑制剂。
不同于蒽环类抗生素和蒽二酮,匹杉琼直接烷基化形成稳定的DNA加合物以及交叉链断裂。另外,由于结合了单杂原子进入环结构以及不具有酮基,匹杉琼不可能生成活性氧化产物,结合离子以及形成可能引起蒽环类抗生素的心脏毒性的醇类代谢产物。
由于这种独特的结构,与多柔比星和米托蒽醌相比,匹杉琼在动物模型上产生了最低程度的心脏毒性。 注射用匹杉琼于2012年5月10日获准作为单一疗法用于治疗多次复发或难治性的侵袭性B细胞非霍奇金淋巴瘤,商品名为Pixuvri,是欧盟首个被批准用于该病人群体的药物。
Pixuvri的上市申请主要基于一项临床III期临床研究PIX 301,研究结果显示患者对Pixuvri联合利妥昔单抗(rituximab)方案的完全应答率较标准方案吉西他滨联合利妥昔单抗更高(分别为20%和6%)。同时,治疗组的无疾病进展期也较对照组长(分别平均为10.2个月和7.6个月)。临床研究中最常见的不良反应为骨髓抑制,即白细胞、血小板和红细胞水平低下,感染的情况较为普遍,但严重的很少。
英文版说明书
Pixuvri: new option in the treatment of non-Hodgkin's B-cell lymphomaPixuvri (pixantrone) is indicated as monotherapy in the treatment of multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphoma.The licensed adult dose of pixantrone is 50mg/m2 by intravenous infusion on days 1, 8 and 15 of each 28-day cycle for up to six cyclesPHARMACOLOGYPixantrone is a cytotoxic aza-anthracenedione. It has shown minimal cardiotoxicity in animal models compared with doxorubicin or mitoxantrone.1CLINICAL STUDIESSalvage treatmentThe safety and efficacy of pixantrone were eva luated in a phase III open-label trial involving 140 patients with aggressive non-Hodgkin's lymphoma who had relapsed after two or more previous chemotherapy regimens including at least one standard anthracycline-containing regimen with a response that lasted at least 24 weeks.2Patients were randomised to receive pixantrone (85mg/m2 by intravenous infusion over one hour on days 1, 8 and 15 of a 28-day cycle for up to six cycles, n=70) or their investigator’s choice of comparator agent at pre-specified standard doses and schedules (n=70).2 Comparator agents included vinorelbine, oxaliplatin, ifosfamide, etoposide, mitoxantrone, gemcitabine and rituximab.2Complete responsesIn the ITT population, significantly more patients achieved a complete or unconfirmed complete response at the end of treatment with pixantrone than with a comparator agent (14 [20%, 95% CI 11.4-31.3] vs 4 [5.7%, 95% CI 1.6-14.0], p=0.021).2In addition, the overall number of responses (complete, unconfirmed complete or partial) was significantly greater in the pixantrone group than in the comparator group (26 [37.1%, 95% CI 25.9-49.5] vs 10 [14.3%, 95% CI 7.1-24.7]; p=0.003).2Median progression-free survival was significantly longer in the pixantrone group than in the comparator group (5.3 months vs 2.6 months, p=0.005).2Median overall survival was also greater in the pixantrone group than in the comparator group although the difference was not significant.2Safety profileThe most commonly observed grade 3 or 4 adverse events in the pixantrone group were neutropenia, leucopenia and thrombocytopenia, all of which occurred at a greater rate than in the comparator group.2The incidence of cardiac adverse events was also higher in the pixantrone group than in the comparator group (35.3% vs 20.9%, respectively); however, these were predominantly asymptomatic grade 1 or 2 declines in left ventricular ejection fraction and did not increase with increasing pixantrone exposure.
药店国别:
产地国家:瑞士
处方药:是
所属类别: 29毫克/毫升/瓶
包装规格: 29毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Cell Therapeutics
原产地英文商品名:Pixuvri 29MG/ML
原产地英文药品名:pixantrone dimaleate
中文参考商品译名:Pixuvri冻干粉注射剂 29毫克/毫升/瓶
中文参考药品译名:马来酸匹杉琼
曾用名:
简介:
马来酸匹杉琼冻干粉注射剂Pixuvri(pixantrone dimaleate)马来酸匹杉琼(pixantrone dimaleate)是一种细胞毒性氮杂蒽二酮。和已经批准的蒽环类抗生素(多柔比星及其他)以及蒽二酮(米托蒽醌)不同,匹杉琼是一种弱拓扑异构酶II抑制剂。
不同于蒽环类抗生素和蒽二酮,匹杉琼直接烷基化形成稳定的DNA加合物以及交叉链断裂。另外,由于结合了单杂原子进入环结构以及不具有酮基,匹杉琼不可能生成活性氧化产物,结合离子以及形成可能引起蒽环类抗生素的心脏毒性的醇类代谢产物。
由于这种独特的结构,与多柔比星和米托蒽醌相比,匹杉琼在动物模型上产生了最低程度的心脏毒性。 注射用匹杉琼于2012年5月10日获准作为单一疗法用于治疗多次复发或难治性的侵袭性B细胞非霍奇金淋巴瘤,商品名为Pixuvri,是欧盟首个被批准用于该病人群体的药物。
Pixuvri的上市申请主要基于一项临床III期临床研究PIX 301,研究结果显示患者对Pixuvri联合利妥昔单抗(rituximab)方案的完全应答率较标准方案吉西他滨联合利妥昔单抗更高(分别为20%和6%)。同时,治疗组的无疾病进展期也较对照组长(分别平均为10.2个月和7.6个月)。临床研究中最常见的不良反应为骨髓抑制,即白细胞、血小板和红细胞水平低下,感染的情况较为普遍,但严重的很少。
英文版说明书
Pixuvri: new option in the treatment of non-Hodgkin's B-cell lymphomaPixuvri (pixantrone) is indicated as monotherapy in the treatment of multiply relapsed or refractory aggressive non-Hodgkin's B-cell lymphoma.The licensed adult dose of pixantrone is 50mg/m2 by intravenous infusion on days 1, 8 and 15 of each 28-day cycle for up to six cyclesPHARMACOLOGYPixantrone is a cytotoxic aza-anthracenedione. It has shown minimal cardiotoxicity in animal models compared with doxorubicin or mitoxantrone.1CLINICAL STUDIESSalvage treatmentThe safety and efficacy of pixantrone were eva luated in a phase III open-label trial involving 140 patients with aggressive non-Hodgkin's lymphoma who had relapsed after two or more previous chemotherapy regimens including at least one standard anthracycline-containing regimen with a response that lasted at least 24 weeks.2Patients were randomised to receive pixantrone (85mg/m2 by intravenous infusion over one hour on days 1, 8 and 15 of a 28-day cycle for up to six cycles, n=70) or their investigator’s choice of comparator agent at pre-specified standard doses and schedules (n=70).2 Comparator agents included vinorelbine, oxaliplatin, ifosfamide, etoposide, mitoxantrone, gemcitabine and rituximab.2Complete responsesIn the ITT population, significantly more patients achieved a complete or unconfirmed complete response at the end of treatment with pixantrone than with a comparator agent (14 [20%, 95% CI 11.4-31.3] vs 4 [5.7%, 95% CI 1.6-14.0], p=0.021).2In addition, the overall number of responses (complete, unconfirmed complete or partial) was significantly greater in the pixantrone group than in the comparator group (26 [37.1%, 95% CI 25.9-49.5] vs 10 [14.3%, 95% CI 7.1-24.7]; p=0.003).2Median progression-free survival was significantly longer in the pixantrone group than in the comparator group (5.3 months vs 2.6 months, p=0.005).2Median overall survival was also greater in the pixantrone group than in the comparator group although the difference was not significant.2Safety profileThe most commonly observed grade 3 or 4 adverse events in the pixantrone group were neutropenia, leucopenia and thrombocytopenia, all of which occurred at a greater rate than in the comparator group.2The incidence of cardiac adverse events was also higher in the pixantrone group than in the comparator group (35.3% vs 20.9%, respectively); however, these were predominantly asymptomatic grade 1 or 2 declines in left ventricular ejection fraction and did not increase with increasing pixantrone exposure.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 匹杉琼冻干粉注射剂Pixuvri(pixantrone dimaleate)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 匹杉琼冻干粉注射剂Pixuvri(pixantrone dimaleate)