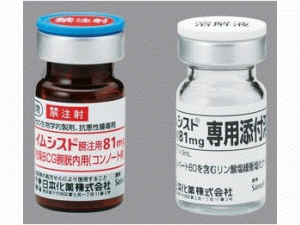
卡介苗免疫治疗剂IMMUCYST 81mg
 药店国别:
产地国家:日本
处方药:是
所属类别: 81毫克(3毫升)/瓶
包装规格: 81毫克(3毫升)/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Sanofi Co.Ltd.
原产地英文商品名:IMMUCYST(イムシスト膀注用)81mg(3mL)/via
原产地英文药品名:Bacillus of Calmette and Guerin(BCG)・Connaught strain
中文参考商品译名:IMMUCYST灌注(イムシスト膀注用)81毫克(3毫升)/瓶
中文参考药品译名:卡介苗免疫治疗剂
曾用名:
简介:部份中文卡介苗免疫治疗剂处方资料(仅供参考)效率分类名称其他生物制品抗肿瘤药物批准日期:2003年10月商標名IMMUCYST一般名BCG・コンノート株Bacillus of Calmette and Guerin(BCG)・Connaught strain性 状它是一种减毒的牛结核杆菌结核分枝杆菌活菌,它是一种酸性杆菌,显示出代码形成。审批条件为了进一步阐明该药在国内的有效性和安全性,在日本进行适当的上市后临床试验药效药理1.
抗肿瘤作用
(1) 通过将小鼠膀胱移行细胞癌细胞系MB49或MBT-2与该药物混合并皮下移植来抑制肿瘤生长和植入。
(2) 在通过用盐酸处理小鼠的膀胱内壁制备的小鼠膀胱癌模型中观察到存活的显着延长,并且在癌细胞植入膀胱后2天将MB49细胞注射入膀胱。2. 作用机序尽管作用的明确机制尚未阐明,但是该药物通过细胞外基质蛋白纤连蛋白的粘附1)被肿瘤细胞,巨噬细胞等吸收并激活免疫活性细胞如巨噬细胞和T淋巴细胞 要做。 这些活化的免疫活性细胞直接损伤癌细胞并分泌抗肿瘤细胞因子(肿瘤坏死因子,干扰素-γ等)并破坏癌细胞。由于该药物不直接对人膀胱癌细胞显示细胞杀伤作用,推测如上所述诱导免疫反应显示对膀胱癌细胞的抗肿瘤作用。
适应病症
浅表性膀胱癌,膀胱上皮内癌
用法与用量
加入3毫升裂解溶液附加到1瓶(81毫克)的产品,制成均匀的悬浮液,进一步用40毫升日本药典盐水稀释,制备一个统一的卡介苗稀释液。浅表性膀胱癌,膀胱上皮内癌将尿道导管在无菌条件下插入膀胱,在排出残余尿液后缓慢注入BCG稀释剂,并尽可能将其保持在膀胱中2小时。 通常每周重复一次,持续8周。浅表性膀胱癌将尿道导管在无菌条件下插入膀胱,在排出残余尿液后缓慢注入BCG稀释剂,并尽可能将其保持在膀胱中2小时。 在经尿道膀胱肿瘤切除术后至少连续14天,每周一次,连续6周,以及连续3周,6,12和18个月,从开始给药开始第3,6,12和18个月,每周一次,连续3周。 根据病人的情况,酌情给药。
存储方法
·到期日期等保存方法储存在2〜8°C避光有效期限自认证之日起两年(小瓶和外箱上的最后生效日期)
包装规格
81毫克:1瓶(附:解液)制造商赛诺菲有限公司注
卡介苗免疫治疗剂英文版说明书
Adjustment and addition of dosage of adjuvant therapy after TURBT for superficial bladder cancer of imcyst® for bladder cancerAbout additional indication of anti-malignant agent 'imsist® for bladder 81 mg'On August 20, 2010, sanofi-aventis Co., Ltd. (Headquarters: Shinjuku-ku, Tokyo, President: Patrick Shoka, hereinafter "sanofi-aventis") and Nippon Kayaku Co., Ltd. (Headquarters: President and Chief Executive Officer, Chiyoda- : Nippon Kayaku, hereinafter referred to as "Nippon Kayaku") is an anti-cancer agent sold by sanofi-aventis "imsist® 81 mg for bladder" (generic name: dry BCG for intravesical (Connaught stock), manufacturing approval: Nippon Kayaku) announced today that we have received additional approval of dosage and dose at adjunctive therapy after transurethral bladder tumor resection (TURBT) in superficial bladder cancer.Imsist®is an antineoplastic agent containing as active ingredient BCG·Connaught stock developed by the Connaught Institute of Canada (currently sanofi pasteur tool: vaccine business department of sanofi·aventis group). In Japan, in October 2002 Nippon Kayaku obtained manufacturing and marketing approval for the efficacy and efficacy of superficial bladder cancer and carcinoma in the bladder epithelium. After that, we conducted a clinical trial to add dosage and dose at adjuvant therapy after transurethral resection in superficial bladder cancer, and applied in April 2009. With this additional approval, ImSyst ® enables maintenance injection therapy in addition to BCG induction therapy for bladder cancer patients with high risk of recurrence.ImSyst ® has been used for treatment of patients with superficial bladder cancer and bladder intraepithelial carcinoma since its release in 2003 in Japan. In addition, adjuvant therapy by imsist after TURBT has been approved in 52 countries around the world as an effective treatment for preventing the recurrence and development of bladder cancer.
药店国别:
产地国家:日本
处方药:是
所属类别: 81毫克(3毫升)/瓶
包装规格: 81毫克(3毫升)/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:Sanofi Co.Ltd.
原产地英文商品名:IMMUCYST(イムシスト膀注用)81mg(3mL)/via
原产地英文药品名:Bacillus of Calmette and Guerin(BCG)・Connaught strain
中文参考商品译名:IMMUCYST灌注(イムシスト膀注用)81毫克(3毫升)/瓶
中文参考药品译名:卡介苗免疫治疗剂
曾用名:
简介:部份中文卡介苗免疫治疗剂处方资料(仅供参考)效率分类名称其他生物制品抗肿瘤药物批准日期:2003年10月商標名IMMUCYST一般名BCG・コンノート株Bacillus of Calmette and Guerin(BCG)・Connaught strain性 状它是一种减毒的牛结核杆菌结核分枝杆菌活菌,它是一种酸性杆菌,显示出代码形成。审批条件为了进一步阐明该药在国内的有效性和安全性,在日本进行适当的上市后临床试验药效药理1.
抗肿瘤作用
(1) 通过将小鼠膀胱移行细胞癌细胞系MB49或MBT-2与该药物混合并皮下移植来抑制肿瘤生长和植入。
(2) 在通过用盐酸处理小鼠的膀胱内壁制备的小鼠膀胱癌模型中观察到存活的显着延长,并且在癌细胞植入膀胱后2天将MB49细胞注射入膀胱。2. 作用机序尽管作用的明确机制尚未阐明,但是该药物通过细胞外基质蛋白纤连蛋白的粘附1)被肿瘤细胞,巨噬细胞等吸收并激活免疫活性细胞如巨噬细胞和T淋巴细胞 要做。 这些活化的免疫活性细胞直接损伤癌细胞并分泌抗肿瘤细胞因子(肿瘤坏死因子,干扰素-γ等)并破坏癌细胞。由于该药物不直接对人膀胱癌细胞显示细胞杀伤作用,推测如上所述诱导免疫反应显示对膀胱癌细胞的抗肿瘤作用。
适应病症
浅表性膀胱癌,膀胱上皮内癌
用法与用量
加入3毫升裂解溶液附加到1瓶(81毫克)的产品,制成均匀的悬浮液,进一步用40毫升日本药典盐水稀释,制备一个统一的卡介苗稀释液。浅表性膀胱癌,膀胱上皮内癌将尿道导管在无菌条件下插入膀胱,在排出残余尿液后缓慢注入BCG稀释剂,并尽可能将其保持在膀胱中2小时。 通常每周重复一次,持续8周。浅表性膀胱癌将尿道导管在无菌条件下插入膀胱,在排出残余尿液后缓慢注入BCG稀释剂,并尽可能将其保持在膀胱中2小时。 在经尿道膀胱肿瘤切除术后至少连续14天,每周一次,连续6周,以及连续3周,6,12和18个月,从开始给药开始第3,6,12和18个月,每周一次,连续3周。 根据病人的情况,酌情给药。
存储方法
·到期日期等保存方法储存在2〜8°C避光有效期限自认证之日起两年(小瓶和外箱上的最后生效日期)
包装规格
81毫克:1瓶(附:解液)制造商赛诺菲有限公司注
卡介苗免疫治疗剂英文版说明书
Adjustment and addition of dosage of adjuvant therapy after TURBT for superficial bladder cancer of imcyst® for bladder cancerAbout additional indication of anti-malignant agent 'imsist® for bladder 81 mg'On August 20, 2010, sanofi-aventis Co., Ltd. (Headquarters: Shinjuku-ku, Tokyo, President: Patrick Shoka, hereinafter "sanofi-aventis") and Nippon Kayaku Co., Ltd. (Headquarters: President and Chief Executive Officer, Chiyoda- : Nippon Kayaku, hereinafter referred to as "Nippon Kayaku") is an anti-cancer agent sold by sanofi-aventis "imsist® 81 mg for bladder" (generic name: dry BCG for intravesical (Connaught stock), manufacturing approval: Nippon Kayaku) announced today that we have received additional approval of dosage and dose at adjunctive therapy after transurethral bladder tumor resection (TURBT) in superficial bladder cancer.Imsist®is an antineoplastic agent containing as active ingredient BCG·Connaught stock developed by the Connaught Institute of Canada (currently sanofi pasteur tool: vaccine business department of sanofi·aventis group). In Japan, in October 2002 Nippon Kayaku obtained manufacturing and marketing approval for the efficacy and efficacy of superficial bladder cancer and carcinoma in the bladder epithelium. After that, we conducted a clinical trial to add dosage and dose at adjuvant therapy after transurethral resection in superficial bladder cancer, and applied in April 2009. With this additional approval, ImSyst ® enables maintenance injection therapy in addition to BCG induction therapy for bladder cancer patients with high risk of recurrence.ImSyst ® has been used for treatment of patients with superficial bladder cancer and bladder intraepithelial carcinoma since its release in 2003 in Japan. In addition, adjuvant therapy by imsist after TURBT has been approved in 52 countries around the world as an effective treatment for preventing the recurrence and development of bladder cancer.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡介苗免疫治疗剂IMMUCYST 81mg
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 卡介苗免疫治疗剂IMMUCYST 81mg