注射用脑蛋白水解物(Cerebrolysin)说明书
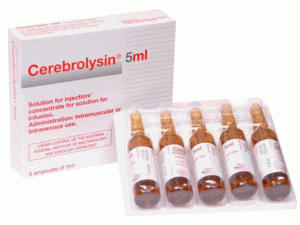 产地国家:奥地利
处 方 药:是
所属类别:5毫升/安醅 5安醅/盒
包装规格:5毫升/安醅 5安醅/盒
计价单位:盒
生产厂家中文参考译名:EVER Neuro Pharma GmbH Austria
生产厂家英文名:EVER Neuro Pharma GmbH Austria
原产地英文商品名:Cerebrolysin Injection 5ml/Ampul 5Ampul/box
原产地英文药品名:Cerebroprotein Hydrolysate
中文参考商品译名:施普善注射剂 5毫升/安醅 5安醅/盒
中文参考药品译名:脑蛋白水解物
产地国家:奥地利
处 方 药:是
所属类别:5毫升/安醅 5安醅/盒
包装规格:5毫升/安醅 5安醅/盒
计价单位:盒
生产厂家中文参考译名:EVER Neuro Pharma GmbH Austria
生产厂家英文名:EVER Neuro Pharma GmbH Austria
原产地英文商品名:Cerebrolysin Injection 5ml/Ampul 5Ampul/box
原产地英文药品名:Cerebroprotein Hydrolysate
中文参考商品译名:施普善注射剂 5毫升/安醅 5安醅/盒
中文参考药品译名:脑蛋白水解物
简介
部份中文脑蛋白水解物处方资料(仅供参考) 使用说明:Cerebrolysin 【中文名稱】活血素 【英文名稱】Cerebrolysin 【類別】神經系統疾病藥物 【別名】 腦蛋白水解物 ,腦活素,活血素 【外文名】Cerebrolysin 【適應症】 用於腦血管病、腦動脈硬化、腦外傷後遺症、腦軟化、中風後遺症、大腦發育不全、癡呆或老年性癡呆、以記憶力衰退為主要表現的神經衰弱等。顱腦手術後、腦震盪後遺症、頑固性抑鬱及癲痛等亦可用。 【用量用法】 靜脈注射:每次10ml。常用靜滴,以本品10~30ml加入等滲鹽水250ml中緩慢滴注,1~2小時滴完。開始時每日1次,隨後每週2~3次或每日1次滴注,連用8~10日。可根據病情,10~20次為1療程。皮下注射:每次2ml;肌肉注射每次5ml。 【注意事項】 1.一般對本品能很好耐受。如注射速度過快,可能引起發熱,偶有過敏反應,如惡寒寒戰等。 2.嚴重腎功能不全者及孕婦禁用。 3.過敏體質者慎用。 【規格】 針劑:5ml、10、1ml。 【储存条件】 储存在温度不高于25°C的黑暗处, 小心保护儿童。 注意:安瓶/小瓶打开后,应立即使用溶液。 【保质期】 安瓿保质期-5年。 瓶保质期-4年。英文版说明书
Cerebrolysin® 10mlx5ampules Cerebrolysin® 10mlx5vials cerebrolysin® 1ml x 10ampules •Manufactured by: EVER Neuro Pharma GmbH Austria Cerebrolysin®: Cerebrolysin® is recommended in cases of organic brain dysfunction, metabolic and neurodegenerative especially senile dementia of the Alzheimer type. In cases of post-stroke complications Cerebrolysin® will speed up the recovery period. Head trauma, post-operative cerebral contusion or concussion require Cerebrolysin®. Cerebrolysin® active compound: Hydrolyzed protein of porcine brain. Cerebrolysin® ingredients: 1 (one)ml solution for injection/infusion containing hydrolysed protein from porcine brain in the form of concentrated solution, 215.2 mg and excipients: sodium hydroxide, water for injections. Cerebrolysin® contraindications - Hypersensitivity to the hydrolyzate of porcine brain protein or any of the excipients. - Epilepsy - Severe renal impairment. Adverse reactions: In rare cases, Cerebrolysin® treatment effects were associated with: - Agitation (aggression, confusion, insomnia) - Hyperventilation, hypertonia, hypotonia, fatigue, tremor, depression, apathy, dizziness and flu symptoms (eg colds, cough, upper respiratory tract infections). During treatment with Cerebrolysin® reported one case of attack type "grand mal" seizures and - Gastrointestinal disorders such as loss of appetite, dyspepsia, diarrhea, constipation, vomiting and nausea - If too rapid injection may be a sensation of warmth or sweating, dizziness and, in isolated cases, palpitations or arrhythmias - Local reactions at the injection site, such as irritation, itching or burning sensation - In rare cases have been observed hypersensitivity or allergic reactions such as skin reactions, local reactions vascular headache, neck pain, pain in limb, fever, back pain, chills and shock. Cerebrolysin® is used in the elderly because the side effects mentioned above are typical for this population and can be observed in the absence of drug use. Directions to use the Cerebrolysin® : May be given single doses up to 50ml/BW (10,760 mg of Cerebrolysin®) but cures treatment is preferable. A cure best treatment involves administering Cerebrolysin® daily for 10-20 days. Recommended Cerebrolysin® daily dose: - Organic brain dysfunction, metabolic and neurodegenerative (dementia) is recommended daily dose of 5-30ml/BW (1076-6456 mg of hydrolyzed porcine brain protein). - Complications post-stroke: the recommended daily dose is 10-50 ml/BW (2152-10760 mg of Cerebrolysin®). - Head trauma, post-operative cerebral contusion or concussion: Recommended daily dose of 10-50ml/BW(2152-10760 mg of Cerebrolysin®). - Children: The recommended daily dose is 1-2ml/BW (215.2 to 430.4 mg of Cerebrolysin®). Cerebrolysin® efficacy can be increased by repeating cures, until no longer see improvements in your health. After the initial cure, Cerebrolysin® dose frequency can be reduced to 2-3 times a week. Must be left free intervals between courses, their duration is equal to the cure. May be given doses up to 5ml/ BW (1076mg of Cerebrolysin®) and intramuscularly to 10 ml/BW (2152 mg of Cerebrolysin®) intravenously. Larger doses of 10-50ml/BW (2152 up to a max. of 10,760 mg of Cerebrolysin®) are recommended only as a slow intravenous infusion after Cerebrolysin® dilution with infusion solutions recommended standard. The infusion must be between 15 and 60 minutes. The following standard Cerebrolysin® infusion solutions were tested for 24 hours at room temperature in the presence of light and were declared compatible: - Sodium chloride solution 0.9% (9 mg Naci/ml) - Ringer solution (Na 153.98 mmol/l, Ca 2.74mmol/l, K>4.02mmol/l, Cl 163.48mmol/l) - Glucose 5% Vitamins and cardiovascular drugs can be administered concomitantly with Cerebrolysin®, but should not be mixed in the syringe. Cerebrolysin® is not addictive and doesn't requires special precautions. Animal studies have shown reproductive toxicity. However, no studies have been performed in humans. Thus, during pregnancy and breastfeeding Cerebrolysin® should be used only after careful eva luation of the risk/benefit. Please avoid to buy Cerebrolysin® from unreliable sellers or manufacturers because it is a very sensitive product and an expired lot could lead to more damage than actual support. The original brand name Cerebrolysin® is Made in Austria. Cerebrolysin® box of 5 brown glass vials with breaking point 10 ml solution for injection/infusion.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 注射用脑蛋白水解物(Cerebrolysin)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 注射用脑蛋白水解物(Cerebrolysin)说明书