巴氯芬隆鞘内注射液(Baclofen )说明书
 产地国家:日本
处 方 药:是
所属类别:10毫克/5毫升/安培 1安培/盒
包装规格:10毫克/5毫升/安培 1安培/盒
计价单位:盒
生产厂家中文参考译名:第一三共制药
生产厂家英文名:Daiichi Sankyo Co.Ltd.
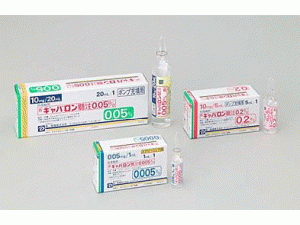
原产地英文商品名:Gabalon(ギャバロン髄注0.2%)10mg/5ml/Ampul 1Ampul/box
原产地英文药品名:Baclofen
中文参考商品译名:Gabalon(ギャバロン髄注0.2%)10毫克/5毫升/安培 1安培/盒
中文参考药品译名:巴氯芬
产地国家:日本
处 方 药:是
所属类别:10毫克/5毫升/安培 1安培/盒
包装规格:10毫克/5毫升/安培 1安培/盒
计价单位:盒
生产厂家中文参考译名:第一三共制药
生产厂家英文名:Daiichi Sankyo Co.Ltd.
原产地英文商品名:Gabalon(ギャバロン髄注0.2%)10mg/5ml/Ampul 1Ampul/box
原产地英文药品名:Baclofen
中文参考商品译名:Gabalon(ギャバロン髄注0.2%)10毫克/5毫升/安培 1安培/盒
中文参考药品译名:巴氯芬
简介
部份中文巴氯芬处方资料(仅供参考) 英文名:Baclofen 商品名:GABALON 中文名:巴氯芬隆鞘内注射 生产商:日本第一三共制药 ギャバロン髄注0.005% 1mL/ギャバロン髄注0.05% 20mL/ギャバロン髄注0.2% 5mL 药用分类名称:抗痉挛剂 批准日期:2005年9月 商標名:GABALON INTRATHECAL INJECTION 一般名:バクロフェン(Baclofen) 化学名:(3RS)-4-Amino-3-(4-chlorophenyl)butanoic acid 分子式:C10H12ClNO2 分子量:213.66 性状 它是白色至黄白色结晶粉末。容易溶于醋酸(100),不易溶于水,极难溶于甲醇或乙醇(95),几乎不溶于二乙醚。溶解在稀盐酸中。 批准条件 采取适当措施,包括在交付本药之前,在交付该药之前,应事先进行培训(包括与泵系统有关的事项),以便充分了解本剂的安全性和有效性,并仅由具有良好治疗知识和经验的医生使用。 药效药理 巴氯芬是β-氨基丁酸(GABA)的衍生物,抑制脊髓的单突触和多突触反射,β-是一种抗痉挛剂,可降低运动神经元的活性。 1. 脊髓反射的抑制作用 抑制脊髓单突触反射和多突触反射的反射,特别是单突触反射被观察到更强烈地抑制。这些反射抑制作用是持续的(脑腔内给药:大鼠),兔子,静脉注射:大鼠,小鸡,猫,体外:青蛙。 2. 运动神经元活性的抑制作用 β - 观察到持续抑制运动神经元活性(静脉注射:大鼠),猫。另外,在脊髓反射和运动神经元的抑制剂量中,观察到肌肉主轴和神经肌肉结的外周作用。 3. 实验性硬收缩的抑制作用 上山-下山除脑硬质收缩(β-硬收缩)和贫血性外脑硬质收缩(α-固缩)均被观察到在剂量依赖性中抑制固縮(脑腔内给药:大鼠、静脉注射:大鼠、猫)。 4. 肌电图改进效果 在遗传性痉挛性大鼠中观察到,肌电图活性在剂量依赖性上被抑制(脑腔内给药和腹腔内给药)。 其他动态延伸反射和诱导肌电图方法在痉挛性麻痹患者中进行了研究,观察到减少和H波恢复曲线(两者都是口服给药)的改善作用, 如克洛努斯. 5. 镇痛作用 当通过压力刺激方法检查时,疼痛阈值增加,观察到镇痛作用(脑腔内给药:大鼠),猫,猴子,腹腔内给药:小鼠,大鼠。 适应症 脑脊髓疾病引起的严重痉挛性麻痹(仅在现有治疗无效的情况下) 用法与用量 脑脊髓疾病引起的严重痉挛性麻痹(仅在现有治疗无效的情况下) 筛选 [检查效果] 为了在种植专用泵系统之前检查该剂的效果,进行筛选。 使用脑注射0.005%(0.05mg/1mL)进行筛选。 通常,成人每天用巴博泰西法(泵)施用50μg作为巴氯芬[骨髓铸造0.005%1mL(1管)],在1-8小时后确认抗痉挛作用。如果未观察到预期效果,则从第一次给药24小时后,75μg[髓重投0.005%为1.5mL(1.5管)]在1至8小时后通过骨髓内给药进行增大确认效果。如果未观察到预期效果,则从第二次给药到24小时后,100μg[髓重投0.005%2mL(2管)]在1-8小时后通过脑腔内给药以同样的方式在1至8小时后确认效果。如果 100μg 未观察到效果,则不成为此剂的治疗对象。 通常,儿童每天25μg作为巴氯芬[0.5mL(0.5管)0.5mL(0.5管)作为巴氯芬,通过巴博泰西方法(泵送)在1-8小时后进行脑腔内给药,在1-8小时后确认抗痉挛作用。然而,体格,但可以增加考虑症状,初始剂量的上限为50μg[脑注0.005%为1mL(1管)]。如果未观察到预期效果,则当初始剂量小于50μg时为50μg,在50μg时增加75μg,在1-8小时后通过脑腔内给药确认效果。如果预期效果未观察到,则根据成人的使用和剂量增加,并在1-8小时后通过脑腔内给药确认效果。如果100μg未观察到效果,则不成为此剂的治疗对象。 设置适当的剂量 在专用泵系统种植后设置适当的剂量,使用脑注0.05%(10mg/20mL)或髓料0.2%(10mg/5mL)。脑本料0.2%可在0.05~0.2%的范围内用日生理盐溶液稀释。 包装 注射液0.005% (1mL) 1安培 注射液 0.05% (20mL) 1安培 注射液 0.2% (5mL) 1安培 提携先 メドトロニック社(米国) 制造供应商 第一三共株式会社 注:以上中文资料不够完整,使用者以原处方资料为准。英文版说明书
GABALON INTRATHECAL INJECTION 0.2%(Baclofen) Brand name :GABALON INTRATHECAL INJECTION 0.2% Active ingredient:Baclofen Dosage form:Injection Print on wrapping: Effects of this medicine This medicine relaxes muscle tone by suppressing synaptic reflex of spinal cord and decreasing activity of γ-motor neuron. It is usually used to treat spastic paralysis(severe) caused by cerebrospinal disease. Before using this medicine, be sure to tell your doctor and pharmacist If you have previously experienced any allergic reactions(itch, rash, etc.)to any medicines. If you have infections before implantation of pump system. If you have or have a history of epilepsy. If you have psychiatric disorders, peptic ulcer, decrease in renal function, liver disorder, respiratory failure, a history of abnormal autonomic reflex. or low body weight. If you are pregnant or breastfeeding. If you are taking any other medicinal products.(Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule(How to take this medicine) Your dosing schedule prescribed by your doctor is((to be written by a healthcare professional)) In general, implant a pumping system dedicated to medicine and injected the pumping system into the medullary cavity once a daily. If your response is confirmed by screening, the dose may be adjusted according to your symptoms until the dosage is adequate up to 60 days after pump implantation. After 61 days of implantation, the treatment will be continued according to your symptoms as a maintenance phase. Precautions while taking this medicine Possible adverse reactions to this medicine The most commonly reported adverse reactions include headache, somnolence, convulsive seizure, hypotonia, numbness, lethargy, coma, difficulty walking, decreased blood pressure, pneumonia, nausea(vomiting), abdominal bloating, constipation, diarrhea (fecal incontinence), urinary disorder, urinary incontinence, urinary retention, rash, fever, lassitude, abnormal feeling, dizziness (light-headedness), pain and muscle weakness.If any of these symptoms occur, consult with your doctor or pharmacist The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately. maintain use despite attempts to discontinue use of certain substances[dependent] The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above. Injection Published: 12/2019 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 巴氯芬隆鞘内注射液(Baclofen )说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 巴氯芬隆鞘内注射液(Baclofen )说明书