托吡酯缓释胶囊说明书_Trokendi_topiramate
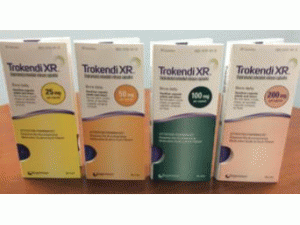
产地国家:美国
处 方 药:是
所属类别:200毫克/粒 30粒/瓶
包装规格:200毫克/粒 30粒/瓶
计价单位:瓶
原产地英文商品名:Trokendi XR 200mg/capsules 30capsules/bottles
原产地英文药品名:topiramate extended-release
中文参考商品译名:Trokendi缓释胶囊 200毫克/粒 30粒/瓶
中文参考药品译名:托吡酯 缓释胶囊,用于口服
作用机制:
托吡酯发挥其抗惊厥和偏头痛预防作用的确切机制尚不清楚; 然而,临床前研究揭示了四种可能有助于托吡酯治疗癫痫和偏头痛预防效果的特性。电生理学和生物化学证据表明,托吡酯,
在药理学相关浓度下,阻断电压依赖性钠通道,增强神经递质γ-氨基丁酸在GABA-A受体的某些亚型中的活性,拮抗谷氨酸受体的AMPA /红藻氨酸亚型,并抑制碳酸酐酶,特别是酶I和IV。
适应症和用法
TROKENDIRR®适用于:
•单药治疗癫痫:6岁及以上患者的初始单药治疗年龄较大的部分发作或原发性全身性强直-阵挛性发作。
•辅助治疗癫痫:6岁患者的辅助治疗部分发病,原发性全身性强直-阵挛性发作,或与Lennox-Gastaut综合征(LGS)相关的癫痫发作。
•偏头痛:预防成人和青少年偏头痛12年龄和年龄剂量和给药。
初始剂量 滴定 推荐的剂量
单药治疗:部分发作或原发性全身强直-阵挛性发作
成人和小儿科 口服50毫克 增加剂量每周一次 400毫克一次
的患者10岁。 每天一次 增量为50毫克前4周
然后100毫克第5周到
第6周
小儿科的患者 25毫克/天 滴定超过5到7周 每日剂量基于重量
6至不到10岁 每晚第一周
辅助治疗
成年部分发病 25毫克至50毫升 增加剂量 200毫克至400毫克一次
癫痫发作或LGS 口服每天一次 增量为25毫克
达到50毫克
有效剂量
成年人一般性强 25毫克至50毫 增加剂量 每日400毫克
直阵挛癫痫发作 升每天服一次 每周一次
有效剂量 增量为25毫克至50毫克
小儿科的患者6年 25毫克一次夜 增加剂量1 每日一次5毫克/千克至9毫克/千克
和更老的部分发 间(基于a范围 周或2周间
病癫痫发作, 1mg/kg至3mg/kg 隔的增量毫
一般性强直阵 一次每日)首先周克/千克至3
挛癫痫发作, 毫克/千克剂或LGS 量滴定应该引导通过临床结果
偏头痛 预防偏头痛 25mg一次日常 增加剂量 每日100毫克
头痛 管理第一周 每周25mg
递增到
达到预期
胶囊整个完好无损。不要撒在食物上,咀嚼或压碎
剂量形式和强度
缓释胶囊:25mg,50mg,100mg和200mg
禁忌症:
•最近使用酒精,即在使用TROKENDILR®之前6小时和之后6小时。
•伴有代谢性酸中毒的患者同时服用二甲双胍
警告和注意事项:
•急性近视和继发性闭角型青光眼:未经治疗的高眼压可导致永久性视力丧失。如果发生,请停止使用TROKENDIRR®
•视野缺损:据报道,这些缺陷与升高的眼压有关。考虑停止使用TROKENDI XR
•低水化和体温过高:监测出汗减少和体温升高,尤其是儿科患者
•代谢性酸中毒:测量血清碳酸盐的基线和定期测量。如果临床适当,考虑减少剂量或停用TROKENDIXR®
•自杀行为和构思:抗癫痫药物会增加自杀行为或构思的风险
•认知/神经精神病:TROKENDIRR®可能导致认知功能障碍。操作包括汽车在内的机械时要小心。
可能会出现抑郁和情绪问题
•胎儿毒性:怀孕期间使用托吡酯可导致唇裂和/或腭裂,并增加胎龄小的风险
•退出AED:TROKENDILR®的撤销应逐步撤销
•高氨血症和脑病:患有先天性代谢紊乱或线粒体活性降低的患者可能会增加高氨血症的风险。如果脑病症状发生,则测量氨
•肾结石:避免与其他碳酸酐酶抑制剂,引起代谢性酸中毒的其他药物或生酮饮食患者一起使用
•体温过低:报告同时使用丙戊酸
不良反应:
最常见的(在单药治疗和辅助治疗中比安慰剂或低剂量邻苯二甲酸酯的频率高10%以上)儿童患者的不良反应为感觉异常,厌食,体重减轻,言语障碍/相关言语问题,疲劳,头晕,嗜睡,神经过敏,精神运动放慢,视力异常,记忆困难,注意力集中注意力不集中,发烧。
在成人和青少年控制的偏头痛临床试验中,最常见的(比安慰剂频率≥5%)不良反应是感觉异常,厌食,体重减轻,记忆困难,味觉变态,上呼吸道感染,腹痛,腹泻,感觉减退,和恶心。
药物相互作用
•口服避孕药:避孕效果降低,出血量增加,尤其是每天剂量超过200毫克。
•苯妥英或卡马西平:同时给予托吡酯降低托吡酯的血浆浓度。
•锂:与高剂量的托吡酯共同给药时监测锂含量。
用于特定人群:
•肾功能损害:(肌酐清除率小于70mL/min/1.73m2),推荐成人剂量的一半。
•接受血液透析的患者:通过血液透析清除托吡酯。需要调整剂量以避免血液透析过程中托吡酯血浆浓度迅速下降。
•儿童使用:因为胶囊必须整个吞服,并且可能不会洒在食物上,压碎或咀嚼,TROKENDIRR®仅限6岁及以上的儿童使用
包装提供/存储和处理:
TROKENDIRR®胶囊
TROKENDIRR®(托吡酯)缓释胶囊可作为缓释胶囊使用
以下优势和颜色:
瓶子:
25毫克(浅绿色不透明体/黄色不透明帽)托吡酯缓释胶囊(黑色印刷品“SPN”和“25”) - 30支装(NDC-17772-101-30)和100支装(NDC-17772-)101-01)
50毫克(浅绿色不透明体/橙色不透明盖)托吡酯缓释胶囊(黑色印刷品“SPN”和“50”) - 30支装(NDC-17772-102-30)和100支装(NDC-17772-)102-01)
100毫克(绿色不透明的身体/蓝色不透明帽)托吡酯缓释胶囊(黑色打印“SPN”和“100”)- 30计数瓶(NDC-17772-103-30)和100计数(NDC-17772-103)-01)
200毫克(粉红色不透明体/蓝色不透明帽)托吡酯缓释胶囊(黑色印刷品“SPN”和“200”)- 30瓶(NDC-17772-104-30)和100计数瓶(NDC-17772-104)-01)
吸塑包装:
25毫克(浅绿色不透明体/黄色不透明帽)托吡酯缓释胶囊(黑色印刷品“SPN”和“25”)- 30支吸塑包装(NDC-17772-101-15)
50毫克(浅绿色不透明体/橙色不透明帽)托吡酯缓释胶囊(黑色印花“SPN”和“50”) - 30支吸塑包装(NDC-17772-102-15)
100毫克(绿色不透明体/蓝色不透明帽)托吡酯缓释胶囊(黑色印花“SPN”和“100”) - 30支吸塑包装(NDC-17772-103-15)
200毫克(粉红色不透明体/蓝色不透明帽)托吡酯缓释胶囊(黑色印花“SPN”和“200”) - 30支吸塑包装(NDC-17772-104-15)
存储和处理:
TROKENDIRR®(托吡酯)缓释胶囊应储存在密闭容器中,温度控制在室温[25°C(77°F);偏差15°C-30°C(59°F-86°F)]。防潮保湿。
TROKENDI XR(topiramate) extended-release capsules
IMPORTANT SAFETY INFORMATION
Do not take Trokendi XR if you have recently consumed or plan to consume alcohol (i.e., within 6 hours prior to and 6 hours after Trokendi XR use)
Swallow Trokendi XR capsules whole. Do not sprinkle on food, chew, or crush.
What are the possible side effects of Trokendi XR?
Trokendi XR can cause serious side effects, including: Eye problems. Serious eye problems include sudden decrease in vision with or without eye pain or redness, a blockage of fluid that may cause increased pressure in the eye (secondary angle closure glaucoma). Call your healthcare provider right away if you have new eye symptoms, including any new problems with your vision.
Decreased sweating and increased body temperature (fever). People, especially children, should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition.
Increased levels of acid in the blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones, can slow the rate of growth in children, and may possibly harm the unborn child of pregnant patients.
High levels of ammonia in the blood. High ammonia in the blood can affect mental activities, slow alertness, cause tiredness, or cause vomiting. Blood ammonia levels have been shown to rise when Trokendi XR is taken with a medicine called valproic acid (e.g., DEPAKENE® and DEPAKOTE®).
Kidney stones. Drink plenty of fluids when taking Trokendi XR to decrease your chances of getting kidney stones.
Low body temperature. Taking Trokendi XR when you are also taking valproic acid may cause a drop in body temperature to less than 95°F, tiredness, confusion, or coma.
Effects on thinking and alertness. Trokendi XR may affect how you think, and can cause confusion and problems with concentration, attention, memory, or speech. Trokendi XR may cause depression or mood problems, tiredness, and sleepiness.
Dizziness or loss of muscle coordination.
The most common side effects include tingling of the arms and legs (paresthesia), not feeling hungry, nausea, weight loss, abnormal vision, a change in the way foods taste, nervousness, speech problems, dizziness, slow reactions, upper respiratory tract infection, sleepiness, diarrhea, pain in abdomen and difficulty with memory. These are not all the possible side effects of Trokendi XR. For more information, ask your healthcare provider or pharmacist.
Like other antiepileptic drugs, Trokendi XR may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Before you take Trokendi XR, tell your healthcare provider if you have or have had depression, mood problems, or suicidal thoughts or behavior. Call a healthcare provider right away if you have thoughts about suicide or dying; have attempted to commit suicide; have new or worsening depression or anxiety; feel agitated or restless; experience panic attacks, trouble sleeping (insomnia), or new or worsening irritability; feel or act more aggressive, angry, or violent; act on dangerous impulses; have an extreme increase in activity and talking (mania); or experience other unusual changes in behavior or mood.
Before taking Trokendi XR, tell your healthcare provider about any other medical conditions, including if you have kidney problems, kidney stones, or are getting kidney dialysis; have a history of metabolic acidosis (too much acid in the blood); have liver problems; have weak, brittle or soft bones (osteomalacia, osteoporosis, osteopenia, or decreased bone density); have lung or breathing problems; have eye problems, especially glaucoma; have diarrhea; have a growth problem; are on a diet high in fat and low in carbohydrates, which is called a ketogenic diet; are having surgery; are pregnant or plan to become pregnant; or if you are breastfeeding. Trokendi XR passes into your breast milk. Breastfed babies may be sleepy or have diarrhea. Talk to your healthcare provider about the best way to feed your baby if you take Trokendi XR.
Trokendi XR can harm your unborn baby. If you take Trokendi XR during pregnancy, your baby has a higher risk for the birth defects of cleft lip, cleft palate, and being smaller than expected at birth. These defects can begin early in pregnancy, even before you know you are pregnant. The long term effects of this are unknown.
Tell your healthcare provider about any other medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Trokendi XR and other medicines may affect each other, causing side effects. Especially tell your healthcare provider if you take valproic acid (e.g., DEPAKENE or DEPAKOTE); any medicines that impair or decrease your thinking, concentration, or muscle coordination; or birth control pills. Trokendi XR may make your birth control pills less effective.
Do not stop Trokendi XR without first talking to a healthcare provider. If you have epilepsy and you stop taking Trokendi XR suddenly, you may have seizures that do not stop. Your healthcare provider will tell you how to stop taking Trokendi XR slowly.
Do not drive a car or operate heavy machinery until you know how Trokendi XR affects you. Trokendi XR can slow your thinking and motor skills, and may affect vision.
INDICATION
Trokendi XR® (topiramate) extended-release capsules are used to prevent migraine headaches in adults and adolescents 12 years and older.
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 托吡酯缓释胶囊说明书_Trokendi_topiramate