索坦胶囊SUTENT 37.5MG CAP(Sunitinib Malate)
 药店国别:
产地国家:美国
处方药:是
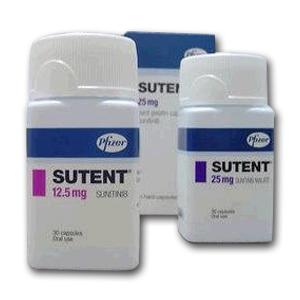
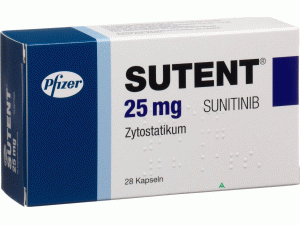
所属类别: 12.5毫克/胶囊 28胶囊/盒
包装规格: 12.5毫克/胶囊 28胶囊/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:SUTENT 37.5MG CAP 28/EA
原产地英文药品名:SUNITINIB MALATE
中文参考商品译名:索坦胶囊 37.5毫克/粒 28粒/瓶
中文参考药品译名:苹果酸舒尼替尼
药店国别:
产地国家:美国
处方药:是
所属类别: 12.5毫克/胶囊 28胶囊/盒
包装规格: 12.5毫克/胶囊 28胶囊/盒
计价单位:盒
生产厂家中文参考译名:
生产厂家英文名:Pfizer
原产地英文商品名:SUTENT 37.5MG CAP 28/EA
原产地英文药品名:SUNITINIB MALATE
中文参考商品译名:索坦胶囊 37.5毫克/粒 28粒/瓶
中文参考药品译名:苹果酸舒尼替尼
简介
部份中文索坦处方资料(仅供参考) 商品名:SUTENT 英文名:Sunitinib Malate 中文名:索坦胶囊(苹果酸舒尼替尼) 药理类别:抗癌药物 妇用药分级 D 级:在对照的人体研究试验中显示该药物对胚胎有不良影响,若此药能带来之效益远超过其它药物的使用,因此即使在其危险性的存在下,仍可接受此药物用于怀孕妇女上。 结构式:N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamideUpToDate UpToDate 药理作用 SUNITINIB是一种能够抑制多种酪氨酸激酶受体的小分子化合物,其中一些受体与肿瘤生长、病理性血管生成和癌症转移有关,它可以通过抑制血管内皮生长因子受体、血小板衍生生长因子受体、干细胞因子受体、Fms-样酪氨酸激酶-3、集落刺激因子受体I型和神经胶质细胞系衍生神经营养因子受体的酪氨酸激酶受体的活性,阻断肿瘤细胞生长的血液和营养供应,从而产生抗血管生成和抗肿瘤作用,此外,Sutent还可以直接杀伤肿瘤细胞。 适应症 SUTENT适用于imatinib mesylate治疗期间出现疾病恶化或对该药出现不能忍受之肠胃道间质肿瘤;治疗晚期或转移性肾细胞癌(病理上为亮细胞癌(clear cell carcinoma))。 用法用量 每天4粒,一次顿服4粒,服用1疗程后需停药观察2周后继续服用。一个疗程6周。药动力学suntinib口服吸收,服药后6-12 h达到血药浓度峰值,t1/2约为40~60 h。重復给药后,suntinib的浓度会增加3-4倍,在10~14 d达到稳态血药浓度。在第14天,suntinib及其主要代谢活性产物的总浓度在62.9~101µg/L之间。主要代谢物t1/2为80~110h,重復给药后体内浓度可蓄积7~10倍。 食物对于suntinib的生物利用度没有影响,本品及其主要代谢物的血浆蛋白结合率分别是95% 和90%,在100~4,000µg/L内没有浓度依赖性。suntinib的表观分佈容积是2,230L。当给药剂量在25-100mg之间时,药一时曲线下面积(AUC)和峰浓度(pmax)均随给药量而成比例增加。 suntinib主要透过细胞色素P450(CYP3A4)代谢。主要活性代谢产物佔全部药物的23%~24%。摄入剂量的61%经粪便排除,16%经肾排除,清除率约为34~62L/h,病人之间的变异性约为40% 。胃肠道间质细胞瘤和恶性肾细胞瘤等实体瘤患者的药动学与健康受试者相似。 药动学 分析显示,年龄、体重、性别、种族、肌酐清除率等对本品及代谢物的药动学无影响。 副作用 常见不良反应包括疲劳、腹泻、食欲减煺、噁心、胃炎、呕吐、腹痛。必要时可适当给予止泻药或止吐药。约1/3的病人会出现皮肤变色。交互作用。 禁忌 对suntinib或者药品中其他成分过敏者禁用。 给付规定 9.31.Sunitinib(如Sutent): 1.肠胃道间质肿瘤:(1)限用于以imatinib治疗期间出现疾病恶化或对该药出现不能忍受之肠胃道间质肿瘤。(2)若使用本药品出现疾病恶化或无法忍受其副作用,不得替换使用imatinib治疗。(3)需经事前审查核准后使用,送审时须检送病歷及对imatinib耐受性不良或无效之证明。 2.晚期肾细胞癌:(1)可用于第一线治疗晚期或转移性肾细胞癌,即病理上为亮细胞癌(clear cell renal carcinoma)。(2)无效后则不给付temsirolimus及其他酪胺酸激?阻断剂(tyrosine kinase inhibitor, TKI)。(3)需检送影像资料,每叁个月评估一次。(4)病人若对药物产生耐受性不佳(intolerance),则以塬来药物减量为塬则,若严重耐受性不佳,可以换其他TKI。 3.进展性,无法切除或转移性分化良好之胰臟神经内分泌肿瘤的成人病患,须同时符合下列条件:(101/5/1)(1)符合WHO 2010分类方式之G1 or G2胰臟神经内分泌瘤。(2)于一年内影像检查证实有明显恶化者。(3)不可合併使用化学治疗或相关标靶药物。(4)经事前专案审查核准后使用,且需每3个月评估一次。 注意事项 suntinib的妊娠期用药安全性为D类。对于孕妇和準备怀孕者,需要告知suntinib对胚胎发育可能存在潜在危害。服药期间哺乳妇女宜暂停哺乳。过量处理suntinib无特效解毒剂。过量时以催吐或导泻等一般解救措施为宜。 药品保存方式 药品应置于摄氏15 ~ 25度乾燥处所;如发生变质或过期,不可再食用。英文版说明书
CYP3A4 inducers (eg, carbamazepine, dexamethasone, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John's wort) :May reduce sunitinib levels, decreasing the therapeutic effect.Grapefruit juice :May elevate sunitinib levels, increasing the pharmacologic and adverse effects.Strong CYP3A4 inhibitors (eg, atazanavir, clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) :May elevate sunitinib levels, increasing the pharmacologic and adverse effects.HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use sutent safely and effectively. See full prescribing information for sutent.sutent (sunitinib malate) capsule for oral useInitial U.S. Approval: 2006RECENT MAJOR CHANGESIndications and Usage ( 1.2)7/2006Warnings and Precautions, Left Ventricular Function ( 5.2)7/2006Warnings and Precautions, QT Interval Prolongation ( 5.3)7/2006Warnings and Precautions, Hemorrhagic Events ( 5.4)7/2006Warnings and Precautions, Hypertension ( 5.5)7/2006INDICATIONS AND USAGESUTENT is a multi-kinase inhibitor indicated forthe treatment of gastrointestinal stromal tumor (GIST) after disease progression on or intolerance to imatinib mesylate.the treatment of advanced renal cell carcinoma (RCC).DOSAGE AND ADMINISTRATIONOne 50-mg oral capsule taken once daily, on a schedule of 4 weeks on treatment followed by 2 weeks off (Schedule 4/2). SUTENT may be taken with or without food. Dose increase or reduction of 12.5-mg increments is recommended based on individual safety and tolerability.DOSAGE FORMS AND STRENGTHS12.5-mg capsules25-mg capsules50-mg capsulesCONTRAINDICATIONSNoneWARNINGS AND PRECAUTIONSPregnancy Category D: There are no adequate and well-controlled studies of SUTENT in pregnant women. If the drug is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant while receiving treatment with SUTENT.Left Ventricular Dysfunction: Left ventricular ejection fraction (LVEF) declines have been observed below the lower limit of normal in both GIST and MRCC patients. It is unknown whether patients with concomitant cardiac conditions may be at a higher risk of developing drug-related left ventricular dysfunction. Physicians are advised to weigh this risk against the potential benefits of the drug. These patients should be carefully monitored for clinical signs and symptoms of CHF while receiving SUTENT. Baseline and periodic eva luations of LVEF should also be considered while the patient is receiving SUTENT. In patients without cardiac risk factors, a baseline eva luation of ejection fraction should be considered. In the presence of clinical manifestations of CHF, discontinuation of SUTENT is recommended. The dose of SUTENT should be interrupted and/or reduced in patients without clinical evidence of CHF but with an ejection fraction <50% and >20% below baseline.QT Interval Prolongation: At approximately twice therapeutic concentrations, SUTENT has been shown to prolong the QTcF interval. Torsade de pointe has been observed in <0.1% of SUTENT-exposed patients. SUTENT should be used with caution in patients with a known history of QT interval prolongation, patients who are taking antiarrhythmics, or patients with relevant pre-existing cardiac disease, bradycardia, or electrolyte disturbances. Concomitant treatment with strong CYP3A4 inhibitors, which may increase sunitinib plasma concentrations, should be used with caution and dose reduction of SUTENT should be considered.Hemorrhagic Events: Tumor-related hemorrhage has been observed in patients treated with SUTENT as early as cycle 1 and as late as cycle 6. These events may occur suddenly, and in the case of pulmonary tumors may present as severe and life-threatening hemoptysis or pulmonary hemorrhage. Clinical assessment of these events should include serial complete blood counts (CBCs) and physical examinations.Hypertension: Severe hypertension (>200 mmHg systolic or 110 mmHg diastolic) occurred in 4% of GIST patients on SUTENT and 1% of GIST patients on placebo, 5% of treatment-naïve MRCC patients on SUTENT and 1% on interferon-α (IFN-α), and 6% of cytokine-refractory MRCC patients. Patients should be monitored for hypertension and treated as needed with standard antihypertensive therapy. In cases of severe hypertension, temporary suspension of SUTENT is recommended until hypertension is controlled.ADVERSE REACTIONSMost common all-causality adverse reactions (≥ 20%) are Constitutional (fatigue), Gastrointestinal (diarrhea, nausea, mucositis/stomatitis, vomiting, constipation, and abdominal pain), Cardiac (hypertension), Dermatology (rash, skin discoloration), Neurology (altered taste, headache), Musculoskeletal (arthralgia, myalgia/limb pain), Metabolism/Nutrition (anorexia, asthenia), Hemorrhage/bleeding (bleeding, all sites).Elevated liver function tests, pancreatic enzymes, creatinine, and myelosuppression were more common in GIST patients treated with SUTENT than placebo. Acquired hypothyroidism was noted in 8 patients (4%) on SUTENT versus 1 (1%) on placebo. Common treatment emergent laboratory abnormalities also observed in the MRCC studies included hypophosphatemia, hyperuricemia and increased lipase.To report SUSPECTED ADVERSE REACTIONS, contact Pfizer, INTERACTIONSCYP3A4 Inhibitors: Concurrent administration of sunitinib malate with the strong CYP3A4 inhibitor, ketoconazole, resulted in an increase in exposure after a single dose of sunitinib malate. A dose reduction for SUTENT should be considered when it must be co-administered with strong CYP3A4 inhibitors.CYP3A4 Inducers: Concurrent administration of SUTENT with the strong CYP3A4 inducer, rifampin, resulted in a reduction in exposure after a single dose of SUTENT. A dose increase for SUTENT should be considered when it must be co-administered with CYP3A4 inducers.USE IN SPECIFIC POPULATIONSNursing Mothers: It is not known whether sunitinib or its primary active metabolite is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants, women should be advised against breastfeeding while taking用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 索坦胶囊SUTENT 37.5MG CAP(Sunitinib Malate)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 索坦胶囊SUTENT 37.5MG CAP(Sunitinib Malate)