氯苯唑酸葡甲胺胶囊(tafamidis meglumine)说明书
产地国家:美国 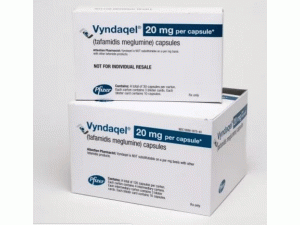 处 方 药:是
所属类别:20毫克/粒 120粒/盒
包装规格:20毫克/粒 120粒/盒
计价单位:盒
生产厂家中文参考译名:辉瑞公司
生产厂家英文名:Pfizer
原产地英文商品名:Vyndaqel 20mg/capsules 120capsules/box
原产地英文药品名:tafamidis meglumine
中文参考商品译名:Vyndaqel胶囊 20毫克/粒 120粒/盒
中文参考药品译名:氯苯唑酸葡甲胺
处 方 药:是
所属类别:20毫克/粒 120粒/盒
包装规格:20毫克/粒 120粒/盒
计价单位:盒
生产厂家中文参考译名:辉瑞公司
生产厂家英文名:Pfizer
原产地英文商品名:Vyndaqel 20mg/capsules 120capsules/box
原产地英文药品名:tafamidis meglumine
中文参考商品译名:Vyndaqel胶囊 20毫克/粒 120粒/盒
中文参考药品译名:氯苯唑酸葡甲胺
 处 方 药:是
所属类别:20毫克/粒 120粒/盒
包装规格:20毫克/粒 120粒/盒
计价单位:盒
生产厂家中文参考译名:辉瑞公司
生产厂家英文名:Pfizer
原产地英文商品名:Vyndaqel 20mg/capsules 120capsules/box
原产地英文药品名:tafamidis meglumine
中文参考商品译名:Vyndaqel胶囊 20毫克/粒 120粒/盒
中文参考药品译名:氯苯唑酸葡甲胺
处 方 药:是
所属类别:20毫克/粒 120粒/盒
包装规格:20毫克/粒 120粒/盒
计价单位:盒
生产厂家中文参考译名:辉瑞公司
生产厂家英文名:Pfizer
原产地英文商品名:Vyndaqel 20mg/capsules 120capsules/box
原产地英文药品名:tafamidis meglumine
中文参考商品译名:Vyndaqel胶囊 20毫克/粒 120粒/盒
中文参考药品译名:氯苯唑酸葡甲胺
简介
新药Vyndaqel(tafamidis meglumine)和Vyndamax(tafamidis)获FDA批准,用于治疗由运甲状腺素介导的淀粉样变性引起的心脏病的新疗法. 近日,美国食品和药物管理局(FDA)批准VYNDAQEL(tafamidis meglumine)和VYNDAMAX(tafamidis)用于治疗成人野生型或遗传性转甲状腺素介导的淀粉样变性(ATTR-CM)的心肌病,以降低心血管死亡率和 心血管相关的住院治疗。 VYNDAQEL和VYNDAMAX是一流的转甲状腺素蛋白稳定剂tafamidis的两种口服制剂,也是FDA批准用于治疗ATTR-CM的第一种和唯一的药物。 批准日期:2019年5月6日 公司:辉瑞 YNDAQEL(tafamidis meglumine)胶囊,用于口服 初始美国批准:2019年 VYNDAMAX TM(tafamidis)胶囊,用于口服给药 初始美国批准:2019年 作用机制 Tafamidis是TTR的选择性稳定剂。Tafamidis在甲状腺素结合位点与TTR结合,稳定化合物并减缓解离成单体,这是淀粉样蛋白形成过程中的限速步骤。 适应症和用法 VYNDAQEL和VYNDAMAX是转甲状腺素蛋白稳定剂,用于治疗野生型或遗传性心肌病转甲状腺素介导的成人淀粉样变性,可降低心血管死亡率和心血管相关住院率。 剂量和用量 推荐剂量为: VYNDAQEL每天口服80毫克,或 VYNDAMAX每天口服61毫克 VYNDAMAX和VYNDAQEL不能以每mg为基础进行替代。 剂量形式和强度 胶囊:Tafamidis葡甲胺20mg和tafamidis 61mg。 禁忌症 无。 用于特定人群 怀孕:根据动物研究,可能会导致胎儿伤害。 哺乳期:建议不要母乳喂养。 包装提供/存储和处理 VYNDAQEL 20mg(tafamidis葡甲胺)软明胶胶囊是黄色,不透明,椭圆形,并用“VYN 20”印刷。 VYNDAQEL胶囊 规格 20毫克 NDC:6609-1975-40 包配置 纸箱的4个中间纸箱。每个中间纸箱包含3个吸塑卡。每个泡罩卡包含10个胶囊。(总共120个胶囊) VYNDAMAX 61毫克(tafamidis)软明胶胶囊为红棕色,不透明,长圆形,并印有 VYNDAMAX胶囊 规格 61毫克 NDC:6609-8730-30 包配置 纸箱的3个吸塑卡。每个吸塑卡含有10粒胶囊。(总共30粒) 将VYNDAQEL和VYNDAMAX储存在20°C至25°C(68°F至77°F)的受控室温下;允许温度为15°C至30°C(59°F至86°F)[参见USP受控室]。温度英文版说明书
U.S. FDA Approves VYNDAQEL® and VYNDAMAX™ for Use in Patients with Transthyretin Amyloid Cardiomyopathy, a Rare and Fatal Disease About ATTR-CM Transthyretin amyloid cardiomyopathy (ATTR-CM) is a rare and fatal condition that is caused by destabilization of a transport protein called transthyretin, which is composed of four identical sub units (a tetramer). When unstable transthyretin tetramers dissociate, they result in misfolded proteins that aggregate into amyloid fibrils and deposit in the heart, causing the heart muscle to become stiff, eventually resulting in heart failure. There are two sub-types of ATTR-CM: hereditary, also known as variant, which is caused by a mutation in the transthyretin gene and can occur in people as early as their 50s and 60s; or with no mutation and associated with aging, known as the wild-type form, which is thought to be more common and usually affects men after age 60. Often ATTR-CM is diagnosed only after symptoms have become severe. Once diagnosed, the median life expectancy in patients with ATTR-CM, dependent on sub-type, is approximately two to 3.5 years. About VYNDAQEL (tafamidis meglumine) and VYNDAMAX(tafamidis) VYNDAQEL (tafamidis meglumine) and VYNDAMAX (tafamidis) are oral transthyretin stabilizers that selectively bind to transthyretin, stabilizing the tetramer of the transthyretin transport protein and slowing the formation of amyloid that causes ATTR-CM. VYNDAMAX 61 mg is a once-daily oral capsule developed for patient convenience. VYNDAQEL and VYNDAMAX are not substitutable on a per milligram basis.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 氯苯唑酸葡甲胺胶囊(tafamidis meglumine)说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 氯苯唑酸葡甲胺胶囊(tafamidis meglumine)说明书