阿法达贝泊汀_Darbepoetin_阿法达贝泊汀注射器「KKF」说明书
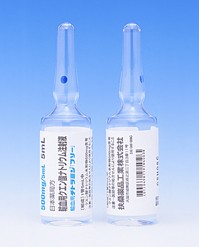
[caption id="attachment_36504" align="alignleft" width="300"] Darbepoetin alfa injection syringe 20mcg(阿法达贝泊汀注射器「KKF」)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:20微克/注射器 10注射器/盒
包装规格:20微克/注射器 10注射器/盒
计价单位:盒
生产厂家中文参考译名:协和麒麟制药
生产厂家英文名:Kyowa Kirin Frontier Co.Ltd.
原产地英文商品名:
Darbepoetin 20mcg/Syringe 10Syringe/box
原产地英文药品名:Darbepoetin Alfa(Genetical Recombination)
中文参考商品译名:Darbepoetin 20微克/注射器 10注射器/盒
中文参考药品译名:阿法达贝泊汀重组
简介:
部份中文阿法达贝泊汀重组处方资料(仅供参考)
英文名:Darbepoetin Alfa
商品名:Darbepoetin
中文名:阿法达贝泊汀重组注射器
生产商:协和麒麟制药
药品简介
近日,由协和麒麟开发的长效促红细胞生成刺激剂药物Darbepoetin Alpha(阿法达贝泊汀重组注射器)在日本推广上市。适用从保守性慢性肾脏疾病到透析的所有肾脏贫血。
药用分类名称:
持续红细胞造血刺激剂制剂
批准日期:2019年6月
欧文商标名:
DARBEPOETIN ALFA INJECTION SYRINGE「KKF」
一般名:Darbepoetin Alfa (Genetical Recombination)
本質:
165个氨基酸残基(C800H1300N228O244S5;分子量:18,cDNA被突变,以改变来自人类肝细胞的红细胞5个氨基酸残基,引入中国仓鼠卵巢细胞产生 176.59)糖蛋白(分子量:约36000)
*达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
处理注意事项:
1. 不要过度操作柱塞杆。此外,在给药后,不要取出后停止。
2. 尽可能在使用前不要从枕头包装中取出注射器。
3. 当注射器尖端的薄膜和尖盖脱落或发现注射器损坏等异常时,不要使用。
4. 稳定性测试
从长期储存试验的达韦波汀阿尔法制剂*使用与最终包装相同的包装形式产品(2~8°C,黑暗,24个月)的结果,该剂在市场流通条件下在规定条件下稳定了两年。
"达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
药效药理:
Darbepoetin α制剂α直接作用于红细胞祖细胞,表现出造血效果。
1. 造血作用
当静脉注射到正常小鼠和大鼠的Darepoetin α制剂中时,与Epoetin α相比,观察到更持久的红细胞造血作用(血红蛋白浓度和网状红细胞计数增加)。此外,在肾性贫血模型大鼠中,通过静脉注射和皮下给药的Darbepoetin α制剂观察到明显的贫血改善。在部分肾切除大鼠中,Darbeepoetin α制剂*表现出与Epoetin α相比的等效贫血改善效果。
2. 作用机制
Darbepoetin α制剂+与红细胞生成素受体结合,促进晚期红细胞祖细胞(CFU-E)和早期红细胞祖细胞(BFU-E)的菌落形成与人类骨髓造血祖细胞(in) 体外)。
适应症:肾性贫血
用法与用量:
<血液透析患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每周静脉注射20μg。
儿童:
通常,作为乙二激素阿尔法(基因重组),每周静脉注射0.33μg/kg(高达20μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Dalveepoetinα(基因重组),每周静脉注射15-60μg。
维持剂量
成人:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)在成人,静脉注射15-60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射30~120μg。
儿童:
一旦获得贫血改善效果,通常作为Darveepoetinα(基因重组)在儿童,静脉注射5~60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射10~120μg。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
<腹膜透析患者及び保存期慢性腎臓病患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次皮下或静脉注射30μg。
儿童:
通常,作为乙二代汀阿尔法(基因重组)在儿童,皮下或静脉给药每2周一次0.5μg/kg(高达30μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次30~120μg皮下或静脉给药。
儿童:
通常,作为乙二激素阿尔法(基因重组)在儿童,皮下或静脉给药10-60μg每2周一次。
维持剂量
成人:
获得贫血改善效果后,通常成人作为Darbeepoetin α(基因重组),每2周皮下或静脉注射30~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周一次60-180μg可以皮下或静脉给药。
儿童:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)的儿童,皮下或静脉给药,每2周一次5~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周10至180μg可以皮下或静脉给药。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
包装:注射器
5微克:10支
10微克:10支
15微克:10支
20微克:10支
30微克:1支,10支
40微克:1支,10支
60微克:1支
120微克:1支
180微克:1支
制造商:协和麒麟有限公司
DARBEPOETIN ALFA INJECTION 180mcg SYRINGE(Darbepoetin alfa(genetical recombination))
Brand name :DARBEPOETIN ALFA INJECTION 180mcg SYRINGE[KKF]
Active ingredient:Darbepoetin alfa (genetical recombination)
Dosage form:injection
Print on wrapping:
Effects of this medicine
This medicine acts on cells that grow into red blood cells (RBCs) to increase the number of RBCs and consequently improves anemia attributable to renal diseases.
It is usually used in the treatment of renal anemia.
Before using this medicine, be sure to tell your doctor and pharmacist
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have a history of myocardial infarction, pulmonary infarction or cerebral infarction.
If you have hypertension.
If you are pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines)
Dosing schedule (How to take this medicine)
Your dosing schedule prescribed by your doctor is ((to be written by a healthcare professional))
For hemodialysis patients: In general, for adults and children, inject intravenously once per week or once every two weeks.
For patients with chronic kidney disease not on dialysis and peritoneal dialysis patients: In general, for adults and children, inject subcutaneously or intravenously once every two or four weeks.
In any case, ask your doctor about the dosing instructions.
This medicine will be administered over a long period under observation of the efficacy.
If this medicine is used for the first time or used after an interval, a lower dose may be administered intravenously or subcutaneously to observe whether any abnormalities occur.
Precautions while taking this medicine
Take iron to acquire adequate efficacy. If iron preparation is prescribed, take as instructed by your doctor.
This medicine may cause hyperkalemia. Follow adequate dietary control.
If you are a patient with chronic kidney disease not on dialysis, seek direction for optimal water intake from your doctor or pharmacist and follow their instruction.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include increased blood pressure, shunt thrombosis/occlusion, headache and malaise. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
paralysis on one side, decreased consciousness, difficulty speaking[cerebral infarction]
decreased consciousness, headache, nausea [cerebral bleeding]
general malaise, yellowing of the white of eyes, loss of appetite[liver dysfunction]
yellowing of the white of eyes, yellowing of the skin, brown urine[jaundice]
dizziness, sudden severe headache, numbness of limbs[hypertensive encephalopathy]
cold sweat, dizziness, decreased consciousness[shock]
swollen eye and lip, palpitations, hives[anaphylaxis]
general malaise, dizziness, shortness of breath[pure red-cell aplasia]
breathing difficulty, sudden pressurized feeling in chest, cold sweat[myocardial infarction]
phlegm with blood, chest pain, breathing difficulty[pulmonary infarction]
Injection
Published: 8/2019
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.
Darbepoetin alfa injection syringe 20mcg(阿法达贝泊汀注射器「KKF」)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:20微克/注射器 10注射器/盒
包装规格:20微克/注射器 10注射器/盒
计价单位:盒
生产厂家中文参考译名:协和麒麟制药
生产厂家英文名:Kyowa Kirin Frontier Co.Ltd.
原产地英文商品名:
Darbepoetin 20mcg/Syringe 10Syringe/box
原产地英文药品名:Darbepoetin Alfa(Genetical Recombination)
中文参考商品译名:Darbepoetin 20微克/注射器 10注射器/盒
中文参考药品译名:阿法达贝泊汀重组
简介:
部份中文阿法达贝泊汀重组处方资料(仅供参考)
英文名:Darbepoetin Alfa
商品名:Darbepoetin
中文名:阿法达贝泊汀重组注射器
生产商:协和麒麟制药
药品简介
近日,由协和麒麟开发的长效促红细胞生成刺激剂药物Darbepoetin Alpha(阿法达贝泊汀重组注射器)在日本推广上市。适用从保守性慢性肾脏疾病到透析的所有肾脏贫血。
药用分类名称:
持续红细胞造血刺激剂制剂
批准日期:2019年6月
欧文商标名:
DARBEPOETIN ALFA INJECTION SYRINGE「KKF」
一般名:Darbepoetin Alfa (Genetical Recombination)
本質:
165个氨基酸残基(C800H1300N228O244S5;分子量:18,cDNA被突变,以改变来自人类肝细胞的红细胞5个氨基酸残基,引入中国仓鼠卵巢细胞产生 176.59)糖蛋白(分子量:约36000)
*达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
处理注意事项:
1. 不要过度操作柱塞杆。此外,在给药后,不要取出后停止。
2. 尽可能在使用前不要从枕头包装中取出注射器。
3. 当注射器尖端的薄膜和尖盖脱落或发现注射器损坏等异常时,不要使用。
4. 稳定性测试
从长期储存试验的达韦波汀阿尔法制剂*使用与最终包装相同的包装形式产品(2~8°C,黑暗,24个月)的结果,该剂在市场流通条件下在规定条件下稳定了两年。
"达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
药效药理:
Darbepoetin α制剂α直接作用于红细胞祖细胞,表现出造血效果。
1. 造血作用
当静脉注射到正常小鼠和大鼠的Darepoetin α制剂中时,与Epoetin α相比,观察到更持久的红细胞造血作用(血红蛋白浓度和网状红细胞计数增加)。此外,在肾性贫血模型大鼠中,通过静脉注射和皮下给药的Darbepoetin α制剂观察到明显的贫血改善。在部分肾切除大鼠中,Darbeepoetin α制剂*表现出与Epoetin α相比的等效贫血改善效果。
2. 作用机制
Darbepoetin α制剂+与红细胞生成素受体结合,促进晚期红细胞祖细胞(CFU-E)和早期红细胞祖细胞(BFU-E)的菌落形成与人类骨髓造血祖细胞(in) 体外)。
适应症:肾性贫血
用法与用量:
<血液透析患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每周静脉注射20μg。
儿童:
通常,作为乙二激素阿尔法(基因重组),每周静脉注射0.33μg/kg(高达20μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Dalveepoetinα(基因重组),每周静脉注射15-60μg。
维持剂量
成人:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)在成人,静脉注射15-60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射30~120μg。
儿童:
一旦获得贫血改善效果,通常作为Darveepoetinα(基因重组)在儿童,静脉注射5~60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射10~120μg。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
<腹膜透析患者及び保存期慢性腎臓病患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次皮下或静脉注射30μg。
儿童:
通常,作为乙二代汀阿尔法(基因重组)在儿童,皮下或静脉给药每2周一次0.5μg/kg(高达30μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次30~120μg皮下或静脉给药。
儿童:
通常,作为乙二激素阿尔法(基因重组)在儿童,皮下或静脉给药10-60μg每2周一次。
维持剂量
成人:
获得贫血改善效果后,通常成人作为Darbeepoetin α(基因重组),每2周皮下或静脉注射30~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周一次60-180μg可以皮下或静脉给药。
儿童:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)的儿童,皮下或静脉给药,每2周一次5~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周10至180μg可以皮下或静脉给药。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
包装:注射器
5微克:10支
10微克:10支
15微克:10支
20微克:10支
30微克:1支,10支
40微克:1支,10支
60微克:1支
120微克:1支
180微克:1支
制造商:协和麒麟有限公司
DARBEPOETIN ALFA INJECTION 180mcg SYRINGE(Darbepoetin alfa(genetical recombination))
Brand name :DARBEPOETIN ALFA INJECTION 180mcg SYRINGE[KKF]
Active ingredient:Darbepoetin alfa (genetical recombination)
Dosage form:injection
Print on wrapping:
Effects of this medicine
This medicine acts on cells that grow into red blood cells (RBCs) to increase the number of RBCs and consequently improves anemia attributable to renal diseases.
It is usually used in the treatment of renal anemia.
Before using this medicine, be sure to tell your doctor and pharmacist
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have a history of myocardial infarction, pulmonary infarction or cerebral infarction.
If you have hypertension.
If you are pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines)
Dosing schedule (How to take this medicine)
Your dosing schedule prescribed by your doctor is ((to be written by a healthcare professional))
For hemodialysis patients: In general, for adults and children, inject intravenously once per week or once every two weeks.
For patients with chronic kidney disease not on dialysis and peritoneal dialysis patients: In general, for adults and children, inject subcutaneously or intravenously once every two or four weeks.
In any case, ask your doctor about the dosing instructions.
This medicine will be administered over a long period under observation of the efficacy.
If this medicine is used for the first time or used after an interval, a lower dose may be administered intravenously or subcutaneously to observe whether any abnormalities occur.
Precautions while taking this medicine
Take iron to acquire adequate efficacy. If iron preparation is prescribed, take as instructed by your doctor.
This medicine may cause hyperkalemia. Follow adequate dietary control.
If you are a patient with chronic kidney disease not on dialysis, seek direction for optimal water intake from your doctor or pharmacist and follow their instruction.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include increased blood pressure, shunt thrombosis/occlusion, headache and malaise. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
paralysis on one side, decreased consciousness, difficulty speaking[cerebral infarction]
decreased consciousness, headache, nausea [cerebral bleeding]
general malaise, yellowing of the white of eyes, loss of appetite[liver dysfunction]
yellowing of the white of eyes, yellowing of the skin, brown urine[jaundice]
dizziness, sudden severe headache, numbness of limbs[hypertensive encephalopathy]
cold sweat, dizziness, decreased consciousness[shock]
swollen eye and lip, palpitations, hives[anaphylaxis]
general malaise, dizziness, shortness of breath[pure red-cell aplasia]
breathing difficulty, sudden pressurized feeling in chest, cold sweat[myocardial infarction]
phlegm with blood, chest pain, breathing difficulty[pulmonary infarction]
Injection
Published: 8/2019
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.
 Darbepoetin alfa injection syringe 20mcg(阿法达贝泊汀注射器「KKF」)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:20微克/注射器 10注射器/盒
包装规格:20微克/注射器 10注射器/盒
计价单位:盒
生产厂家中文参考译名:协和麒麟制药
生产厂家英文名:Kyowa Kirin Frontier Co.Ltd.
原产地英文商品名:
Darbepoetin 20mcg/Syringe 10Syringe/box
原产地英文药品名:Darbepoetin Alfa(Genetical Recombination)
中文参考商品译名:Darbepoetin 20微克/注射器 10注射器/盒
中文参考药品译名:阿法达贝泊汀重组
简介:
部份中文阿法达贝泊汀重组处方资料(仅供参考)
英文名:Darbepoetin Alfa
商品名:Darbepoetin
中文名:阿法达贝泊汀重组注射器
生产商:协和麒麟制药
药品简介
近日,由协和麒麟开发的长效促红细胞生成刺激剂药物Darbepoetin Alpha(阿法达贝泊汀重组注射器)在日本推广上市。适用从保守性慢性肾脏疾病到透析的所有肾脏贫血。
药用分类名称:
持续红细胞造血刺激剂制剂
批准日期:2019年6月
欧文商标名:
DARBEPOETIN ALFA INJECTION SYRINGE「KKF」
一般名:Darbepoetin Alfa (Genetical Recombination)
本質:
165个氨基酸残基(C800H1300N228O244S5;分子量:18,cDNA被突变,以改变来自人类肝细胞的红细胞5个氨基酸残基,引入中国仓鼠卵巢细胞产生 176.59)糖蛋白(分子量:约36000)
*达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
处理注意事项:
1. 不要过度操作柱塞杆。此外,在给药后,不要取出后停止。
2. 尽可能在使用前不要从枕头包装中取出注射器。
3. 当注射器尖端的薄膜和尖盖脱落或发现注射器损坏等异常时,不要使用。
4. 稳定性测试
从长期储存试验的达韦波汀阿尔法制剂*使用与最终包装相同的包装形式产品(2~8°C,黑暗,24个月)的结果,该剂在市场流通条件下在规定条件下稳定了两年。
"达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
药效药理:
Darbepoetin α制剂α直接作用于红细胞祖细胞,表现出造血效果。
1. 造血作用
当静脉注射到正常小鼠和大鼠的Darepoetin α制剂中时,与Epoetin α相比,观察到更持久的红细胞造血作用(血红蛋白浓度和网状红细胞计数增加)。此外,在肾性贫血模型大鼠中,通过静脉注射和皮下给药的Darbepoetin α制剂观察到明显的贫血改善。在部分肾切除大鼠中,Darbeepoetin α制剂*表现出与Epoetin α相比的等效贫血改善效果。
2. 作用机制
Darbepoetin α制剂+与红细胞生成素受体结合,促进晚期红细胞祖细胞(CFU-E)和早期红细胞祖细胞(BFU-E)的菌落形成与人类骨髓造血祖细胞(in) 体外)。
适应症:肾性贫血
用法与用量:
<血液透析患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每周静脉注射20μg。
儿童:
通常,作为乙二激素阿尔法(基因重组),每周静脉注射0.33μg/kg(高达20μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Dalveepoetinα(基因重组),每周静脉注射15-60μg。
维持剂量
成人:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)在成人,静脉注射15-60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射30~120μg。
儿童:
一旦获得贫血改善效果,通常作为Darveepoetinα(基因重组)在儿童,静脉注射5~60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射10~120μg。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
<腹膜透析患者及び保存期慢性腎臓病患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次皮下或静脉注射30μg。
儿童:
通常,作为乙二代汀阿尔法(基因重组)在儿童,皮下或静脉给药每2周一次0.5μg/kg(高达30μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次30~120μg皮下或静脉给药。
儿童:
通常,作为乙二激素阿尔法(基因重组)在儿童,皮下或静脉给药10-60μg每2周一次。
维持剂量
成人:
获得贫血改善效果后,通常成人作为Darbeepoetin α(基因重组),每2周皮下或静脉注射30~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周一次60-180μg可以皮下或静脉给药。
儿童:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)的儿童,皮下或静脉给药,每2周一次5~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周10至180μg可以皮下或静脉给药。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
包装:注射器
5微克:10支
10微克:10支
15微克:10支
20微克:10支
30微克:1支,10支
40微克:1支,10支
60微克:1支
120微克:1支
180微克:1支
制造商:协和麒麟有限公司
DARBEPOETIN ALFA INJECTION 180mcg SYRINGE(Darbepoetin alfa(genetical recombination))
Brand name :DARBEPOETIN ALFA INJECTION 180mcg SYRINGE[KKF]
Active ingredient:Darbepoetin alfa (genetical recombination)
Dosage form:injection
Print on wrapping:
Effects of this medicine
This medicine acts on cells that grow into red blood cells (RBCs) to increase the number of RBCs and consequently improves anemia attributable to renal diseases.
It is usually used in the treatment of renal anemia.
Before using this medicine, be sure to tell your doctor and pharmacist
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have a history of myocardial infarction, pulmonary infarction or cerebral infarction.
If you have hypertension.
If you are pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines)
Dosing schedule (How to take this medicine)
Your dosing schedule prescribed by your doctor is ((to be written by a healthcare professional))
For hemodialysis patients: In general, for adults and children, inject intravenously once per week or once every two weeks.
For patients with chronic kidney disease not on dialysis and peritoneal dialysis patients: In general, for adults and children, inject subcutaneously or intravenously once every two or four weeks.
In any case, ask your doctor about the dosing instructions.
This medicine will be administered over a long period under observation of the efficacy.
If this medicine is used for the first time or used after an interval, a lower dose may be administered intravenously or subcutaneously to observe whether any abnormalities occur.
Precautions while taking this medicine
Take iron to acquire adequate efficacy. If iron preparation is prescribed, take as instructed by your doctor.
This medicine may cause hyperkalemia. Follow adequate dietary control.
If you are a patient with chronic kidney disease not on dialysis, seek direction for optimal water intake from your doctor or pharmacist and follow their instruction.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include increased blood pressure, shunt thrombosis/occlusion, headache and malaise. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
paralysis on one side, decreased consciousness, difficulty speaking[cerebral infarction]
decreased consciousness, headache, nausea [cerebral bleeding]
general malaise, yellowing of the white of eyes, loss of appetite[liver dysfunction]
yellowing of the white of eyes, yellowing of the skin, brown urine[jaundice]
dizziness, sudden severe headache, numbness of limbs[hypertensive encephalopathy]
cold sweat, dizziness, decreased consciousness[shock]
swollen eye and lip, palpitations, hives[anaphylaxis]
general malaise, dizziness, shortness of breath[pure red-cell aplasia]
breathing difficulty, sudden pressurized feeling in chest, cold sweat[myocardial infarction]
phlegm with blood, chest pain, breathing difficulty[pulmonary infarction]
Injection
Published: 8/2019
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.
Darbepoetin alfa injection syringe 20mcg(阿法达贝泊汀注射器「KKF」)[/caption]
药店国别:
产地国家:日本
处 方 药:是
所属类别:20微克/注射器 10注射器/盒
包装规格:20微克/注射器 10注射器/盒
计价单位:盒
生产厂家中文参考译名:协和麒麟制药
生产厂家英文名:Kyowa Kirin Frontier Co.Ltd.
原产地英文商品名:
Darbepoetin 20mcg/Syringe 10Syringe/box
原产地英文药品名:Darbepoetin Alfa(Genetical Recombination)
中文参考商品译名:Darbepoetin 20微克/注射器 10注射器/盒
中文参考药品译名:阿法达贝泊汀重组
简介:
部份中文阿法达贝泊汀重组处方资料(仅供参考)
英文名:Darbepoetin Alfa
商品名:Darbepoetin
中文名:阿法达贝泊汀重组注射器
生产商:协和麒麟制药
药品简介
近日,由协和麒麟开发的长效促红细胞生成刺激剂药物Darbepoetin Alpha(阿法达贝泊汀重组注射器)在日本推广上市。适用从保守性慢性肾脏疾病到透析的所有肾脏贫血。
药用分类名称:
持续红细胞造血刺激剂制剂
批准日期:2019年6月
欧文商标名:
DARBEPOETIN ALFA INJECTION SYRINGE「KKF」
一般名:Darbepoetin Alfa (Genetical Recombination)
本質:
165个氨基酸残基(C800H1300N228O244S5;分子量:18,cDNA被突变,以改变来自人类肝细胞的红细胞5个氨基酸残基,引入中国仓鼠卵巢细胞产生 176.59)糖蛋白(分子量:约36000)
*达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
处理注意事项:
1. 不要过度操作柱塞杆。此外,在给药后,不要取出后停止。
2. 尽可能在使用前不要从枕头包装中取出注射器。
3. 当注射器尖端的薄膜和尖盖脱落或发现注射器损坏等异常时,不要使用。
4. 稳定性测试
从长期储存试验的达韦波汀阿尔法制剂*使用与最终包装相同的包装形式产品(2~8°C,黑暗,24个月)的结果,该剂在市场流通条件下在规定条件下稳定了两年。
"达尔韦波汀阿尔法制剂"是指"达韦波汀阿尔法(重组)制剂(雀巢注射液安慰剂)"。
药效药理:
Darbepoetin α制剂α直接作用于红细胞祖细胞,表现出造血效果。
1. 造血作用
当静脉注射到正常小鼠和大鼠的Darepoetin α制剂中时,与Epoetin α相比,观察到更持久的红细胞造血作用(血红蛋白浓度和网状红细胞计数增加)。此外,在肾性贫血模型大鼠中,通过静脉注射和皮下给药的Darbepoetin α制剂观察到明显的贫血改善。在部分肾切除大鼠中,Darbeepoetin α制剂*表现出与Epoetin α相比的等效贫血改善效果。
2. 作用机制
Darbepoetin α制剂+与红细胞生成素受体结合,促进晚期红细胞祖细胞(CFU-E)和早期红细胞祖细胞(BFU-E)的菌落形成与人类骨髓造血祖细胞(in) 体外)。
适应症:肾性贫血
用法与用量:
<血液透析患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每周静脉注射20μg。
儿童:
通常,作为乙二激素阿尔法(基因重组),每周静脉注射0.33μg/kg(高达20μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Dalveepoetinα(基因重组),每周静脉注射15-60μg。
维持剂量
成人:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)在成人,静脉注射15-60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射30~120μg。
儿童:
一旦获得贫血改善效果,通常作为Darveepoetinα(基因重组)在儿童,静脉注射5~60μg每周一次。 如果每周施用一次贫血改善,则启动剂量为当时一次剂量的两倍,每两周更换一次,每2周可静脉注射10~120μg。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
<腹膜透析患者及び保存期慢性腎臓病患者>
初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次皮下或静脉注射30μg。
儿童:
通常,作为乙二代汀阿尔法(基因重组)在儿童,皮下或静脉给药每2周一次0.5μg/kg(高达30μg)。
• 从红细胞生成素(Epoetin alpha(基因重组)、Epoetin β(基因重组)等)配方中切换初始剂量
成人:
通常,成人作为Darbeepoetinα(基因重组),每2周一次30~120μg皮下或静脉给药。
儿童:
通常,作为乙二激素阿尔法(基因重组)在儿童,皮下或静脉给药10-60μg每2周一次。
维持剂量
成人:
获得贫血改善效果后,通常成人作为Darbeepoetin α(基因重组),每2周皮下或静脉注射30~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周一次60-180μg可以皮下或静脉给药。
儿童:
获得贫血改善效果后,通常,作为Darbeepoetinα(基因重组)的儿童,皮下或静脉给药,每2周一次5~120μg。 如果贫血改善在2周一次维持,作为起始剂量的2倍的剂量,此时的剂量,改变每4周一次,每4周10至180μg可以皮下或静脉给药。
另外,在任何情况下贫血症状的程度,但适当增加或减少的年龄等,最高剂量是180μg一次。
包装:注射器
5微克:10支
10微克:10支
15微克:10支
20微克:10支
30微克:1支,10支
40微克:1支,10支
60微克:1支
120微克:1支
180微克:1支
制造商:协和麒麟有限公司
DARBEPOETIN ALFA INJECTION 180mcg SYRINGE(Darbepoetin alfa(genetical recombination))
Brand name :DARBEPOETIN ALFA INJECTION 180mcg SYRINGE[KKF]
Active ingredient:Darbepoetin alfa (genetical recombination)
Dosage form:injection
Print on wrapping:
Effects of this medicine
This medicine acts on cells that grow into red blood cells (RBCs) to increase the number of RBCs and consequently improves anemia attributable to renal diseases.
It is usually used in the treatment of renal anemia.
Before using this medicine, be sure to tell your doctor and pharmacist
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have a history of myocardial infarction, pulmonary infarction or cerebral infarction.
If you have hypertension.
If you are pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines)
Dosing schedule (How to take this medicine)
Your dosing schedule prescribed by your doctor is ((to be written by a healthcare professional))
For hemodialysis patients: In general, for adults and children, inject intravenously once per week or once every two weeks.
For patients with chronic kidney disease not on dialysis and peritoneal dialysis patients: In general, for adults and children, inject subcutaneously or intravenously once every two or four weeks.
In any case, ask your doctor about the dosing instructions.
This medicine will be administered over a long period under observation of the efficacy.
If this medicine is used for the first time or used after an interval, a lower dose may be administered intravenously or subcutaneously to observe whether any abnormalities occur.
Precautions while taking this medicine
Take iron to acquire adequate efficacy. If iron preparation is prescribed, take as instructed by your doctor.
This medicine may cause hyperkalemia. Follow adequate dietary control.
If you are a patient with chronic kidney disease not on dialysis, seek direction for optimal water intake from your doctor or pharmacist and follow their instruction.
Possible adverse reactions to this medicine
The most commonly reported adverse reactions include increased blood pressure, shunt thrombosis/occlusion, headache and malaise. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
paralysis on one side, decreased consciousness, difficulty speaking[cerebral infarction]
decreased consciousness, headache, nausea [cerebral bleeding]
general malaise, yellowing of the white of eyes, loss of appetite[liver dysfunction]
yellowing of the white of eyes, yellowing of the skin, brown urine[jaundice]
dizziness, sudden severe headache, numbness of limbs[hypertensive encephalopathy]
cold sweat, dizziness, decreased consciousness[shock]
swollen eye and lip, palpitations, hives[anaphylaxis]
general malaise, dizziness, shortness of breath[pure red-cell aplasia]
breathing difficulty, sudden pressurized feeling in chest, cold sweat[myocardial infarction]
phlegm with blood, chest pain, breathing difficulty[pulmonary infarction]
Injection
Published: 8/2019
The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿法达贝泊汀_Darbepoetin_阿法达贝泊汀注射器「KKF」说明书
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿法达贝泊汀_Darbepoetin_阿法达贝泊汀注射器「KKF」说明书