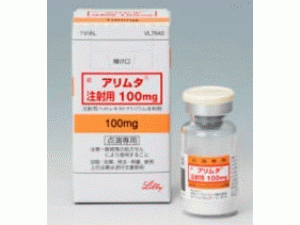
培美曲塞,培美曲塞二钠冻干粉注射剂 アリムタ注射用(Alimta Injection 500mg)
 药店国别:无
产地国家:日本
处方药:是
所属类别: 500毫克/瓶
包装规格: 500毫克/瓶
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:Lilly
原产地英文商品名:Alimta Injection(アリムタ注射用)500mg/Vial
原产地英文药品名:Pemetrexed Sodium Hydrate
中文参考商品译名:力比泰(アリムタ注射用)500毫克/瓶
中文参考药品译名:培美曲塞二钠
曾用名:无
药店国别:无
产地国家:日本
处方药:是
所属类别: 500毫克/瓶
包装规格: 500毫克/瓶
计价单位:瓶
生产厂家中文参考译名:无
生产厂家英文名:Lilly
原产地英文商品名:Alimta Injection(アリムタ注射用)500mg/Vial
原产地英文药品名:Pemetrexed Sodium Hydrate
中文参考商品译名:力比泰(アリムタ注射用)500毫克/瓶
中文参考药品译名:培美曲塞二钠
曾用名:无
简介
部份中文力比泰处方资料(仅供参考) 中文名称:培美曲塞二钠 英文名:Pemetrexed 国外商品名:Alimta 曾用名:MTA,LY231514 类别:化药3.1类 剂型:原料+粉针 规格:500mg/瓶 生产厂家:Eli Lilly(礼来公司上市) 作用机制 Alimta是一种多靶点抗代谢的抗肿瘤药物,为叶酸拮抗剂,可以抑制胸苷酸合成酶、二氢叶酸还原酶、甘氨酸核糖核苷甲酰基转移酶等叶酸依赖性酶,这些酶参与胸腺嘧啶核苷和嘌呤核苷的生物合成,从而达到抗肿瘤的效果。 药代动力学 主要经尿清除。在肾功能正常的患者(肌苷清除率为90 ml/min),Alimta的系统清除率为91.8 ml/min。与顺铂、叶酸、维生素B12联合应用时不影响Alimta的药代动力学。 特殊人群:在26至80岁之间,未发现年龄对Alimta的代谢有影响。无儿童相关资料。药物代谢无性别差异。肝功能障碍者,升高的AST、ALT、胆红素不影响Alimta的代谢。 适应症 Alimta联合顺铂一线治疗不可切除的恶性胸膜间皮瘤。单药二线治疗非小细胞肺癌。已批准和正进行临床试验的适应症。 批准:[美国,适应症为恶性胸膜间皮瘤(和顺铂联用);(美国,适应症为非小细胞肺癌);Ⅲ期临床(美国,适应症为乳腺癌和结肠癌);Ⅱ期临床(美国、英国、加拿大、西班牙等多个国家,适应症为乳腺癌、结肠癌、膀胱癌等多种实体肿瘤。 用法用量 Alimta仅可静脉滴注,与顺铂联用,推荐剂量为500 mg/m2,第1天,滴注超过10分钟,21天为一个周期。顺铂推荐剂量为75 mg/m2,在Alimta滴注结束后30分钟开始滴注,时间超过2小时。肌苷清除率> 45 ml/min的患者不需调整剂量,肌苷清除率<45 ml/min的患者不建议使用Alimta。接受Alimta治疗的患者应同时应用叶酸和维生素B12,可减少治疗相关的血液学毒性和胃肠道毒性。任何疑问,请遵医嘱! 禁忌症 禁用于有严重培美曲唑过敏史的患者不良反应主要不良反应为骨髓抑制,表现为中性粒细胞减少症、血小板减少症和贫血。还有发热、感染、口腔炎/咽炎、皮疹/脱皮。对怀孕妇女可影响胎儿。 培美曲塞二钠项目推荐报告别差异。肝功能障碍者,升高的AST、ALT、胆红素不影响Alimta的代谢。培美曲塞二钠是一种新的、多靶位叶酸拮抗剂,是一种核苷酸合酶/二氢叶酸还原酶双重抑制剂,可同时阻断三种不同的对癌细胞的生存至关重要的酶靶标。FDA继2004年2月份批准了与顺铂联用治疗恶性胸膜间皮瘤之后,又以快速审批途径批准将其作为局部晚期肺癌或转移性非小细胞肺癌的二线治疗药物。2004年8月19日,礼来公司宣布,FDA批准其培美曲塞二钠用于非小细胞肺癌治疗。英文版说明
CANCERApproval Marks Third European Indication for ALIMTAINDIANAPOLIS, IND., USA, 11 April, 2008 – Eli Lilly and Company (NYSE: LLY) today announced that European health authorities have approved the use of ALIMTA® (pemetrexed for injection) for a histologically-based use in the first-line treatment of advanced non-small-cell lung cancer (NSCLC), the most common form of lung cancer. This approval – the third for pe-metrexed in Europe – follows an initial positive opinion issued by the European Medicines Agency’s (EMEA) Committee for Medicinal Products for Human Use (CHMP) on February 21, 2008.The EMEA specifically approved pemetrexed in combination with cisplatin as a first-line treat-ment for NSCLC patients with other than predominantly squamous cell histology. Histology is the microscopic study of tissue and NSCLC is classified by its histology. Previously, all histolo-gies were treated similarly.“This approval opens the door for a novel, tailored approach based on histology or tissue type,” said Richard Gaynor, M.D., vice president of cancer research and global oncology platform leader for Lilly. “Our hope is that this study provides physicians with a powerful tool for choos-ing the right drug for the right patient that leads to optimal treatment results.”The approval in first-line NSCLC is based on a Phase III randomized study that eva luated pemetrexed plus cisplatin versus GEMZAR® (gemcitabine HCl for injection) plus cisplatin. The 1,725-patient study, the largest Phase III clinical trial undertaken in the first-line setting of NSCLC, met its primary endpoint of non-inferiority relative to overall survival.However, when it came to survival by histology, the study found, in a pre-planned histological analysis, that patients with either adenocarcinoma or large-cell carcinoma had a clinically relevant improvement in overall survival when treated with the pemetrexed regimen in the first-line setting. In comparison, patients with squamous cell histology were found to have a more favorable overall survival when treated with the gemcitabine regimen.The lead investigator of the study, Giorgio Scagliotti, M.D., Department of Clinical and Biological Sciences Thoracic Oncology Unit, University of Torino, Orbassano, Italy, said the approval of pemetrexed plus cisplatin in a first-line setting marked an important step forward in treating the world’s leading cause of cancer deaths.“This study provides further evidence of the need to use a tailored approach to treating lung cancer patients, rather than simply using a particular medicine because of the treatment stage,” said Dr. Scagliotti.Data from the first-line NSCLC study was presented at the Presidential Symposium at the 12th World Conference on Lung Cancer (WCLC) in Seoul, Korea, on September 5, 2007, and at the Presidential Symposium III at the 14th Annual European Cancer Conference (ECCO) on September 24, 2007, in Barcelona, Spain.This regulatory approval paves the way for launches in Europe and applies to all 27 countries of the European Union, as well as Norway, Iceland, and Liechtenstein.Pemetrexed as Second-Line NSCLC TreatmentIn regard to the second-line indication for pemetrexed in NSCLC, the EMEA has approved a change in the indication to patients with other than predominantly squamous cell histology. This decision was based on a retrospective analysis of Phase III data in patients treated with either pemetrexed or docetaxel second-line that showed NSCLC patients with other than predominantly squamous cell histology had improved survival when treated with pemetrexed as compared to docetaxel, whereas patients with squamous cell histology treated with docetaxel had improved survival compared to pemetrexed. Data from this trial was presented at ECCO on September 25, 2007, in Barcelona.Notes to EditorAbout ALIMTA (pemetrexed for injection)Pemetrexed is currently indicated in combination with cisplatin in more than 85 countries for the treatment of patients with malignant pleural mesothelioma (MPM) whose disease is unresectable or who are otherwise not candidates for curative surgery. Pemetrexed is also approved as a sec-ond-line, single agent for the treatment of patients with locally advanced or metastatic NSCLC after prior chemotherapy. This latest approval is for pemetrexed in combination with cisplatin as a first-line treatment for NSCLC patients with other than predominantly squamous cell histology.About Non-Small-Cell Lung Cancer (NSCLC)NSCLC is the most common type of lung cancer and represents 85 to 90 percent of all lung can-cers. NSCLC has five-tier staging, starting at 0 and rising to the severity of stage IV. NSCLC can spread through the lymphatic system, penetrating the chest lining, ribs, and the nerves and blood vessels that lead to the arm. The liver, bones and brain are potential targets if the cancer-ous cells enter the bloodstream.According to the World Health Organization (WHO) Cancer Report, lung cancer is the world's most common cancer and the leading cause of cancer death for men and women. More than 1 million people die from lung cancer each year.NSCLC is defined as a group of histologies, that is, tumor types differentiated by cellular struc-ture. The most common NSCLC histology types are squamous (or epidermoid) carcinoma, ade-nocarcinoma, and large-cell carcinoma. These histologies are often classified together because to date, approaches to diagnosis, staging, prognosis, and treatment have been similar.About Lilly Oncology, a Division of Eli Lilly and CompanyFor more than four decades, Lilly Oncology has been collaborating with cancer researchers to deliver innovative treatment choices and valuable programs to patients and their physicians. Inspired by courageous patients living with cancer, Lilly Oncology is providing treatments that are considered global standards of care and developing a broad portfolio of novel targeted therapies to accelerate the pace and progress of cancer care.About Eli Lilly and CompanyLilly, a leading innovation-driven corporation, is developing a growing portfolio of first-in-class and best-in-class pharmaceutical products by applying the latest research from its own worldwide laboratories and from collaborations with eminent scientific organizations. Headquartered in Indianapolis, Ind., Lilly provides answers – through medicines and information – for some of the world's most urgent medical needs.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 培美曲塞,培美曲塞二钠冻干粉注射剂 アリムタ注射用(Alimta Injection 500mg)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 培美曲塞,培美曲塞二钠冻干粉注射剂 アリムタ注射用(Alimta Injection 500mg)