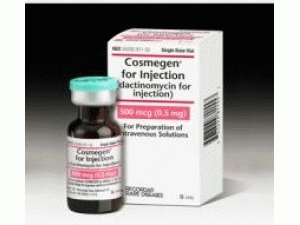
更生霉素放线菌素COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)
 药店国别:
产地国家:德国
处方药:是
所属类别: 0.5毫克/毫升/瓶
包装规格: 0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:ASD/RECORDATI
原产地英文商品名:COSMEGEN VL 0.5MG ASD DS 1
原产地英文药品名:dactinomycin for injection
中文参考商品译名:COSMEGEN注射剂 0.5毫克/瓶 1瓶
中文参考药品译名:更生霉素放线菌素
曾用名:
简介:Cosmegen是一种处方药用于各种类型的癌症化疗。Cosmegen属于一组称为放线菌素的抗生素,用于局部复发或局部区域的姑息性和/或辅助性治疗实体恶性肿瘤。批准日期:1964年12月10日公司:ASD/RECORDATICOSMEGEN(放线菌素[dactinomycin])注射,用于静脉注射最初的美国批准:1964年最近的重大变化推荐用于Wilms肿瘤的。
作用机制
COSMEGEN是一种细胞毒性放线菌素,可以结合DNA并抑制RNA合成。已经在不同人类癌症的动物模型中证明了放线菌素的细胞毒活性。
适应症和用法
COSMEGEN是一种放线菌素,用于治疗:成人和儿童患有肿瘤的Wilms,作为多阶段联合化疗方案的一部分。成人和儿童横纹肌肉瘤患者,作为多阶段联合化疗方案的一部分。Ewing肉瘤的成人和儿童患者,作为多阶段联合化疗方案的一部分。患有转移性,非精原细胞性睾丸癌的成人和儿童患者,作为多阶段联合化疗方案的一部分。妊娠后滋养细胞肿瘤患者,单一药物或化疗方案的组合。具有局部复发或局部区域实体恶性肿瘤的成人患者,具有缓解或辅助区域灌注的成分。
剂量和用量
肾母细胞瘤:作为多药联合化疗方案的一部分,推荐剂量为每3至6周静脉注射45 mcg / kg。横纹肌肉瘤:对于多药联合化疗方案,推荐剂量为15 mcg/ kg静脉内盎司。尤文肉瘤:推荐剂量为1250 mcg / m2静脉注射,每3周一次,共51周,作为多药联合化疗方案的一部分。转移性非精原细胞性睾丸癌:推荐剂量为每3周静脉注射1000 mcg / m2,作为基于顺铂的多药物化疗方案的一部分。妊娠滋养细胞肿瘤:非转移性和低风险转移性疾病:推荐剂量为每天静脉注射12 mcg /kg,持续5天,作为单一药物。高危转移性疾病:作为多药联合化疗方案的一部分,推荐剂量为第1天和第2天静脉注射500mcg,每2周一次,最长8周。局部相关和局部区域固体恶性肿瘤的区域输注:下肢或骨盆:推荐剂量为50mcg/kg,一次用美法仑。上肢:使用美法仑一次推荐剂量为35mcg/kg。剂量形式和强度用于注射:500mcg,作为单剂量小瓶中的冻干粉末。
禁忌症
无。
警告和注意事项
继发性恶性肿瘤或白血病:治疗后继发性恶性肿瘤的风险增加。静脉闭塞性疾病:可引起严重或致命的VOD。监测AST,ALT,总胆红素,肝肿大,体重增加或腹水的升高。考虑延迟下一剂量。外渗:立即打破注射或输液并涂冰。骨髓抑制:在每个周期之前监测血细胞计数。如果严重的骨髓抑制没有改善,则延迟下一次给药。免疫接种:不建议在治疗前或治疗期间接种活病毒疫苗。严重粘膜皮肤反应:停止治疗。肾毒性:经常监测肌酐和电解质。肝毒性:监测转氨酶,碱性磷酸酶和胆红素。辐射毒性和辐射回收的增强:在伴随辐射期间减少50%的剂量。在放射两个月内给药时要小心。胚胎-胎儿毒性:可导致胎儿伤害。告知患者胎儿的潜在风险并使用有效的避孕措施。
不良反应
常见的不良反应是:感染,脱发,皮疹,吞咽困难,疲劳,发烧,恶心,呕吐,贫血,中性粒细胞减少,血小板减少,粘膜炎和肝毒性。
包装提供/存储和处理
用于静脉内使用的COSMEGEN(注射用放线菌素)以单剂量小瓶中的无菌无定形黄色至橙色冻干粉末形式提供。 每个COSMEGEN小瓶(NDC55292-811-55)含有0.5mg的放线菌素和20mg的甘露醇。储存温度为20至25ºC(68至77ºF);允许的偏差在15到30ºC(59到86ºF)之间[见USP受控室温]。COSMEGEN防止光线和湿度。将重构的COSMEGEN在室温下保存不超过4小时,从重建到完成给药[见剂量和给药]。COSMEGEN是一种细胞毒性药物。遵循适用的特殊处理和处理程序。
更生霉素放线菌素冻干粉注射剂英文版说明书
Approval: 1964Indications and UsageCOSMEGEN® (dactinomycin for injection), as part of a combination chemotherapy and/or multi-modality treatment regimen, is indicated for the treatment of Wilms’ tumor, childhood rhabdomyosarcoma, Ewing’s sarcoma and metastatic, nonseminomatous testicular cancer.COSMEGEN is indicated as a single agent, or as part of a combination chemotherapy regimen, for the treatment of gestational trophoblastic neoplasia.COSMEGEN, as a component of regional perfusion, is indicated for the palliative and/or adjunctive treatment of locally recurrent or locoregional solid malignancies. It is extremely important to observe the patient daily for toxic side effects when combination chemotherapy is employed.Important Safety InformationCOSMEGEN should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents. This drug is HIGHLY TOXIC and both powder and solution must be handled and administered with care. Inhalation of dust or vapors and contact with skin or mucous membranes, especially those of the eyes, must be avoided. Avoid exposure during pregnancy. Due to the toxic properties of dactinomycin (e.g., corrosivity, carcinogenicity, mutagenicity, teratogenicity), special handling procedures should be reviewed prior to handling and followed diligently. Dactinomycin is extremely corrosive to soft tissue. If extravasation occurs during intravenous use, severe damage to soft tissues will occur. In at least one instance, this has led to contracture of the arms.COSMEGEN is contraindicated in patients who are hypersensitive to any component of this product. COSMEGEN should not be given at or about the time of infection with chicken pox or herpes zoster.Reports indicate an increased incidence of second primary tumors (including leukemia) following treatment with radiation and antineoplastic agents. Gastrointestinal toxicity and marrow suppression have been reported when combined therapy including COSMEGEN and radiation have been used.Renal, hepatic and bone marrow functions should be assessed frequently. COSMEGEN should be administered to infants only over the age 6 to 12 months. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.Common adverse reactions may include any tissue of the body, including the hematopoietic system resulting in myelosuppression.
药店国别:
产地国家:德国
处方药:是
所属类别: 0.5毫克/毫升/瓶
包装规格: 0.5毫克/毫升/瓶
计价单位:瓶
生产厂家中文参考译名:
生产厂家英文名:ASD/RECORDATI
原产地英文商品名:COSMEGEN VL 0.5MG ASD DS 1
原产地英文药品名:dactinomycin for injection
中文参考商品译名:COSMEGEN注射剂 0.5毫克/瓶 1瓶
中文参考药品译名:更生霉素放线菌素
曾用名:
简介:Cosmegen是一种处方药用于各种类型的癌症化疗。Cosmegen属于一组称为放线菌素的抗生素,用于局部复发或局部区域的姑息性和/或辅助性治疗实体恶性肿瘤。批准日期:1964年12月10日公司:ASD/RECORDATICOSMEGEN(放线菌素[dactinomycin])注射,用于静脉注射最初的美国批准:1964年最近的重大变化推荐用于Wilms肿瘤的。
作用机制
COSMEGEN是一种细胞毒性放线菌素,可以结合DNA并抑制RNA合成。已经在不同人类癌症的动物模型中证明了放线菌素的细胞毒活性。
适应症和用法
COSMEGEN是一种放线菌素,用于治疗:成人和儿童患有肿瘤的Wilms,作为多阶段联合化疗方案的一部分。成人和儿童横纹肌肉瘤患者,作为多阶段联合化疗方案的一部分。Ewing肉瘤的成人和儿童患者,作为多阶段联合化疗方案的一部分。患有转移性,非精原细胞性睾丸癌的成人和儿童患者,作为多阶段联合化疗方案的一部分。妊娠后滋养细胞肿瘤患者,单一药物或化疗方案的组合。具有局部复发或局部区域实体恶性肿瘤的成人患者,具有缓解或辅助区域灌注的成分。
剂量和用量
肾母细胞瘤:作为多药联合化疗方案的一部分,推荐剂量为每3至6周静脉注射45 mcg / kg。横纹肌肉瘤:对于多药联合化疗方案,推荐剂量为15 mcg/ kg静脉内盎司。尤文肉瘤:推荐剂量为1250 mcg / m2静脉注射,每3周一次,共51周,作为多药联合化疗方案的一部分。转移性非精原细胞性睾丸癌:推荐剂量为每3周静脉注射1000 mcg / m2,作为基于顺铂的多药物化疗方案的一部分。妊娠滋养细胞肿瘤:非转移性和低风险转移性疾病:推荐剂量为每天静脉注射12 mcg /kg,持续5天,作为单一药物。高危转移性疾病:作为多药联合化疗方案的一部分,推荐剂量为第1天和第2天静脉注射500mcg,每2周一次,最长8周。局部相关和局部区域固体恶性肿瘤的区域输注:下肢或骨盆:推荐剂量为50mcg/kg,一次用美法仑。上肢:使用美法仑一次推荐剂量为35mcg/kg。剂量形式和强度用于注射:500mcg,作为单剂量小瓶中的冻干粉末。
禁忌症
无。
警告和注意事项
继发性恶性肿瘤或白血病:治疗后继发性恶性肿瘤的风险增加。静脉闭塞性疾病:可引起严重或致命的VOD。监测AST,ALT,总胆红素,肝肿大,体重增加或腹水的升高。考虑延迟下一剂量。外渗:立即打破注射或输液并涂冰。骨髓抑制:在每个周期之前监测血细胞计数。如果严重的骨髓抑制没有改善,则延迟下一次给药。免疫接种:不建议在治疗前或治疗期间接种活病毒疫苗。严重粘膜皮肤反应:停止治疗。肾毒性:经常监测肌酐和电解质。肝毒性:监测转氨酶,碱性磷酸酶和胆红素。辐射毒性和辐射回收的增强:在伴随辐射期间减少50%的剂量。在放射两个月内给药时要小心。胚胎-胎儿毒性:可导致胎儿伤害。告知患者胎儿的潜在风险并使用有效的避孕措施。
不良反应
常见的不良反应是:感染,脱发,皮疹,吞咽困难,疲劳,发烧,恶心,呕吐,贫血,中性粒细胞减少,血小板减少,粘膜炎和肝毒性。
包装提供/存储和处理
用于静脉内使用的COSMEGEN(注射用放线菌素)以单剂量小瓶中的无菌无定形黄色至橙色冻干粉末形式提供。 每个COSMEGEN小瓶(NDC55292-811-55)含有0.5mg的放线菌素和20mg的甘露醇。储存温度为20至25ºC(68至77ºF);允许的偏差在15到30ºC(59到86ºF)之间[见USP受控室温]。COSMEGEN防止光线和湿度。将重构的COSMEGEN在室温下保存不超过4小时,从重建到完成给药[见剂量和给药]。COSMEGEN是一种细胞毒性药物。遵循适用的特殊处理和处理程序。
更生霉素放线菌素冻干粉注射剂英文版说明书
Approval: 1964Indications and UsageCOSMEGEN® (dactinomycin for injection), as part of a combination chemotherapy and/or multi-modality treatment regimen, is indicated for the treatment of Wilms’ tumor, childhood rhabdomyosarcoma, Ewing’s sarcoma and metastatic, nonseminomatous testicular cancer.COSMEGEN is indicated as a single agent, or as part of a combination chemotherapy regimen, for the treatment of gestational trophoblastic neoplasia.COSMEGEN, as a component of regional perfusion, is indicated for the palliative and/or adjunctive treatment of locally recurrent or locoregional solid malignancies. It is extremely important to observe the patient daily for toxic side effects when combination chemotherapy is employed.Important Safety InformationCOSMEGEN should be administered only under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents. This drug is HIGHLY TOXIC and both powder and solution must be handled and administered with care. Inhalation of dust or vapors and contact with skin or mucous membranes, especially those of the eyes, must be avoided. Avoid exposure during pregnancy. Due to the toxic properties of dactinomycin (e.g., corrosivity, carcinogenicity, mutagenicity, teratogenicity), special handling procedures should be reviewed prior to handling and followed diligently. Dactinomycin is extremely corrosive to soft tissue. If extravasation occurs during intravenous use, severe damage to soft tissues will occur. In at least one instance, this has led to contracture of the arms.COSMEGEN is contraindicated in patients who are hypersensitive to any component of this product. COSMEGEN should not be given at or about the time of infection with chicken pox or herpes zoster.Reports indicate an increased incidence of second primary tumors (including leukemia) following treatment with radiation and antineoplastic agents. Gastrointestinal toxicity and marrow suppression have been reported when combined therapy including COSMEGEN and radiation have been used.Renal, hepatic and bone marrow functions should be assessed frequently. COSMEGEN should be administered to infants only over the age 6 to 12 months. Dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range.Common adverse reactions may include any tissue of the body, including the hematopoietic system resulting in myelosuppression.
用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 更生霉素放线菌素COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 更生霉素放线菌素COSMEGEN VL 0.5MG ASD(DACTINOMYCIN)





