泊马度胺胶囊pomalidomide(Pomalyst Capsules 4mg)
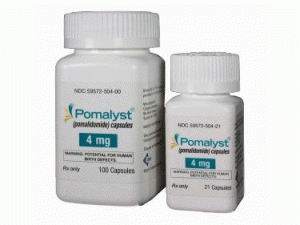 产地国家:美国
处方药:是
所属类别: 4毫克/胶囊 21胶囊/瓶
包装规格: 4毫克/胶囊 21胶囊/瓶
计价单位:瓶
生产厂家英文名:Celgene Corporation
原产地英文商品名:Pomalyst 4mg/capsules 21capsules/bottles
原产地英文药品名:pomalidomide
中文参考商品译名:Pomalyst 4毫克/胶囊 21胶囊/瓶
中文参考药品译名:泊马度胺
产地国家:美国
处方药:是
所属类别: 4毫克/胶囊 21胶囊/瓶
包装规格: 4毫克/胶囊 21胶囊/瓶
计价单位:瓶
生产厂家英文名:Celgene Corporation
原产地英文商品名:Pomalyst 4mg/capsules 21capsules/bottles
原产地英文药品名:pomalidomide
中文参考商品译名:Pomalyst 4毫克/胶囊 21胶囊/瓶
中文参考药品译名:泊马度胺
简介
抗癌新药Pomalidomide(商品名 Pomalyst 美国塞尔基因生产)获美国FDA批准为治疗晚期多发性骨髓瘤FDA 药物评价和研究中心的血液学和肿瘤产品室主任Richard Pazdur,M.D.说:“Pomalyst是免疫调节剂类的第三个药物,包括来那度胺和沙利度胺[thalidomide],和去年批准的第二个治疗 多发性骨髓瘤药物。“对多发性骨髓瘤治疗是调整以符合个体患者需要,和今天的批准为已对其他药物不反应患者提供另一个治疗选择。” 批准日期:2013年2月8日 原研厂家:Celgene CorporationPOMALYST(泊马度胺 pomalidomide)胶囊,用于口服使用 美国首次批准:2013年 警告:胚胎毒性和血管和血管内皮生长因子有关完整的盒装警告,请参阅完整的处方信息胚胎毒性POMALYST在怀孕期间禁忌。 POMALYST是沙利度胺类似物。沙利度胺是一种已知的人类致畸因子,可导致严重威胁生命的严重缺陷。对于生殖潜力的女性:在开始治疗前排除怀孕。在治疗期间通过使用2种可靠的避孕方法预防怀孕。POMALYST仅通过名为POMALYSTREMS®的限制程序提供。静脉血栓栓塞在POMALYST治疗的多发性骨髓瘤患者中发生深静脉血栓形成(DVT),肺栓塞(PE),心肌梗死和中风。推荐使用抗血栓预防。 最近的主要变化用量和管理:06/16 作用机制 番茄红素是沙利度胺的类似物,是具有抗肿瘤活性的免疫调节剂。 在体外细胞测定中,番茄红素抑制增殖并诱导造血肿瘤细胞凋亡。 此外,对于来那度胺敏感型和来那度敏抗性细胞系,罗沙利特抑制了来那度敏多巴胺耐药细胞系的增殖,并与地塞米松协同作用,诱导肿瘤细胞凋亡。 番茄红素增强了T细胞和自然杀伤(NK)细胞介导的免疫,并通过单核细胞抑制促炎细胞因子(例如TNF-α和IL-6)的产生。 Pomalidomide在小鼠肿瘤模型和体外脐带模型中表现出抗血管生成活性。 适用症:POMALYST是一种与地塞米松组合使用的沙利度胺类似物,用于接受至少两种先前疗法的多发性骨髓瘤患者,包括来那度胺和蛋白酶体抑制剂,并且已经证实了在最后一次治疗完成后60天内或之后的疾病进展 用法用量:多发性骨髓瘤:每天口服4mg,每天1-2天,重复28天,直至疾病进展。地塞米松给药参见第14.1节。 剂量形式:胶囊。1mg,2mg,3mg和4mg 禁忌症:怀孕 警告和注意事项: 血液毒性:中性粒细胞减少症是最常见的3/4级不良事件。监测患者的血液毒性,特别是中性粒细胞减少症。 肝毒性:肝功能衰竭,包括死亡;每月监测肝功能检查。 超敏反应:血管性水肿和严重皮肤病反应已有报道。停止POMALYST用于血管性水肿和严重的皮肤病反应。 肿瘤裂解综合征(TLS):监测患有TLS危险的患者(即肿瘤负荷较重的患者),并采取适当的预防措施。 不良反应: 最常见的不良反应(≥30%)包括疲劳和虚弱,中性粒细胞减少症,贫血,便秘,恶心,腹泻,呼吸困难,上呼吸道感染,背痛和发热。 药物相互作用:强CYP1A2抑制剂:避免伴随使用强CYP1A2抑制剂。如果必须使用强烈的CYP1A2抑制剂,则将POMALYST剂量降低50%。 特定人口中使用: 哺乳:停止药物或哺乳,考虑到母亲对药物的重要性。 肾损伤:需要透析的严重肾功能损害患者减少POMALYST剂量至少25%。在血液透析天血液透析后服用POMALYST剂量。 肝损伤:在轻度至中度肝损伤患者中将POMALYST剂量减少25%,在严重肝损伤患者中将POMALYST剂量降低50%。 存储:20°C-25°C(68°F-77°F);偏移允许在15°C-30°C(59°F-86°F)。 [见USP受控室温]。处理和处置在处理POMALYST时应谨慎行事。 POMALYST胶囊不应打开或压碎。如果来自POMALYST的粉末接触皮肤,请立即用肥皂和水彻底清洗皮肤。如果POMALYST接触到粘膜,请用水彻底冲洗干净。遵循适当处理和处置抗癌药物的程序。英文版说明书
POMALYST®(pomalidomide)capsulesPOMALYST®(pomalidomide) is a prescription medicine, taken along with the medicine dexamethasone, used to treat people with multiple myeloma who have previously received at least 2 medicines to treat multiple myeloma, including a proteasome inhibitor and lenalidomide, and whose disease has become worse during treatment or within 60 days of finishing the last treatment. It is not known if POMALYST is safe and effective in children.Important Safety InformationWhat is the most important information I should know about POMALYST?Before you begin taking POMALYST, you must read and agree to all of the instructions in the POMALYST REMS® program. Before prescribing POMALYST, your healthcare provider (HCP) will explain the POMALYST REMS program to you and have you sign the Patient-Physician Agreement Form.POMALYST can cause serious side effects, including:Possible birth defects (deformed babies) or death of an unborn baby. Females who are pregnant or plan to become pregnant must not take POMALYST. –POMALYST is similar to the medicine thalidomide (THALOMID®), which is known to cause severe life-threatening birth defects. POMALYST has not been tested in pregnant females. POMALYST has harmed unborn animals in animal testing.–Females must not get pregnant for at least 4 weeks before starting POMALYST, while taking POMALYST, during any breaks (interruptions) in your treatment with POMALYST, and for at least 4 weeks after stopping POMALYST.–Females who can become pregnant: ◦–Must have pregnancy tests weekly for 4 weeks once treatment has started, then every 4 weeks if your menstrual cycle is regular or every 2 weeks if your menstrual cycle is irregular. If you miss your period or have unusual bleeding, you will need to have a pregnancy test and receive counseling.◦–Must agree to use 2 different forms of effective birth control at the same time, for at least 4 weeks before, while taking, during any breaks (interruptions) in treatment, and for at least 4 weeks after stopping POMALYST. Talk with your HCP to find out about options for acceptable forms of birth control that you may use to prevent pregnancy.–If you become pregnant while taking POMALYST, stop taking it right away and call your HCP. If your HCP is not available, you can call Celgene Customer There is a pregnancy exposure registry that monitors the outcomes of females who take POMALYST during pregnancy, or if their male partner takes POMALYST and they are exposed during pregnancy. You can enroll in this registry by calling Celgene Corporation at the phone number listed above.–POMALYST can pass into human semen. Males, including those who have had a vasectomy, must always use a latex or synthetic condom during any sexual contact with a pregnant female or a female that can become pregnant while taking POMALYST, during any breaks (interruptions) in your treatment with POMALYST, and for at least 4 weeks after stopping POMALYST. ◦–If a female becomes pregnant with your sperm, you should call your HCP right away. The baby may be exposed to POMALYST and may be born with birth defects.◦–Do not have unprotected sexual contact with a female who is or could become pregnant. Tell your HCP if you have unprotected sexual contact with a female who is or could become pregnant.◦–Do not donate sperm while taking POMALYST, during any breaks (interruptions) in your treatment, and for at least 4 weeks after stopping POMALYST.–Do not donate blood while you take POMALYST, during any breaks (interruptions) in your treatment, and for at least 4 weeks after stopping POMALYST. If someone who is pregnant gets your donated blood, her baby may be exposed to POMALYST and may be born with birth defects.Blood clots in your arteries, veins, and lungs; heart attack; and stroke. Most people who take POMALYST will also take a blood thinner medicine to help prevent blood clots. –Before taking POMALYST, tell your HCP if you have had a blood clot in the past, if you have high blood pressure or hyperlipidemia (high level of fat in your blood), or if you smoke. Tell your HCP about all the medicines you take because certain other medicines can also increase your risk for blood clots.–Call your HCP or get medical help right away if you get any of the following during treatment with POMALYST: (1) signs or symptoms of a blood clot in the lung, arm, or leg, including shortness of breath, chest pain, or arm or leg swelling; (2) signs or symptoms of a heart attack, including chest pain that may spread to the arms, neck, jaw, back, or stomach area (abdomen); feeling sweaty; shortness of breath; feeling sick; or vomiting; or (3) signs or symptoms of stroke, including sudden numbness or weakness, especially on one side of the body; severe headache or confusion; or problems with vision, speech, or balance.Who should not take POMALYST?Do not take POMALYST if you are pregnant, plan to become pregnant, or become pregnant during treatment with POMALYST. See "What is the most important information I should know about POMALYST?"What should I tell my healthcare provider (HCP) before taking POMALYST?If you smoke cigarettes (POMALYST may not work as well in people who smoke), have any other medical conditions, or are breastfeeding. Do not breastfeed during treatment with POMALYST—it is not known if POMALYST passes into breast milk and can harm the baby.Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. POMALYST and other medicines may affect each other, causing serious side effects. Talk with your HCP before taking any new medicines.How should I take POMALYST?Take POMALYST exactly as prescribed and follow all the instructions of the POMALYST REMS program.Swallow POMALYST capsules whole with water 1 time a day. Do not break, chew, or open capsules.Take POMALYST at the same time each day with or without food.If you are on hemodialysis, take POMALYST after hemodialysis on hemodialysis days.Do not open POMALYST capsules or handle them any more than needed. If you touch a broken POMALYST capsule or the medicine in the capsule, wash the area of your body right away with soap and water.If you miss a dose of POMALYST and it has been less than 12 hours since your regular time, take POMALYST as soon as you remember. If it has been more than 12 hours, just skip your missed dose. Do not take 2 doses at the same time.If you take too much POMALYST, call your healthcare provider (HCP) right away.Do not share POMALYST with other people. It may cause birth defects and other serious problems.What are the possible side effects of POMALYST?See "What is the most important information I should know about POMALYST?"POMALYST can cause serious side effects, including: –Low white blood cells (neutropenia), low platelets (thrombocytopenia), and low red blood cells (anemia) are common with POMALYST, but can also be serious. You may need a blood transfusion or certain medicines if your blood counts drop too low. Your blood counts should be checked by your healthcare provider (HCP) weekly for the first 8 weeks of treatment and monthly after that.–Severe liver problems, including liver failure or death. Your HCP should do blood tests to check your liver function during your treatment with POMALYST. Tell your HCP right away if you develop any of the following symptoms: yellowing of your skin or the white parts of your eyes (jaundice); dark or brown (tea-colored) urine; pain on the upper right side of your stomach area (abdomen); bleeding or bruising more easily than normal, or feeling very tired.–Severe allergic and skin reactions. Call your HCP if you have any symptoms of an allergic reaction, including: swelling of your lips, mouth, tongue, or throat; trouble breathing; or skin reaction.–Dizziness and confusion. Avoid taking other medicines that may cause dizziness and confusion during treatment with POMALYST. Avoid situations that require you to be alert until you know how POMALYST affects you.–Nerve damage. Stop taking POMALYST and call your HCP if you develop numbness, tingling, pain, or a burning sensation in your hands, legs, or feet.–New cancers (malignancies). New cancers, including certain blood cancers (acute myelogenous leukemia or AML) have been seen in people who received POMALYST. Talk with your HCP about your risk.–Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure and the need for dialysis treatment, abnormal heart rhythm, seizure, and sometimes death. Your HCP may do blood tests to check you for TLS.The most common side effects of POMALYST include tiredness, weakness, constipation, nausea, diarrhea, shortness of breath, upper respiratory tract infection, back pain, and fever.These are not all the possible side effects of POMALYST. Your HCP may tell you to stop taking POMALYST if you develop certain serious side effects during treatment. Call your HCP for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 泊马度胺胶囊pomalidomide(Pomalyst Capsules 4mg)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 泊马度胺胶囊pomalidomide(Pomalyst Capsules 4mg)