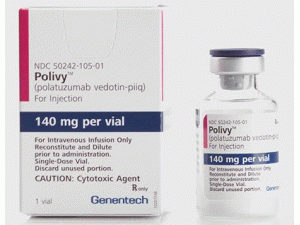
阿扎胞苷注射剂AZACITIDINE(VIDAZA 100MG SDV PWD)
 产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶
包装规格: 100毫克/瓶 1瓶
计价单位:盒
生产厂家英文名:CELGENE CORPORATION
原产地英文商品名:VIDAZA 100MG SDV PWD 1/EA
原产地英文药品名:AZACITIDINE
中文参考商品译名:维达扎 100毫克/瓶 1瓶
中文参考药品译名:阿扎胞苷
曾用名:阿扎胞苷、5-氮杂胞苷、5-氮杂胞嘧啶核苷、氮胞苷、氮杂胞苷、Ladakamycin
产地国家:美国
处方药:是
所属类别: 100毫克/瓶 1瓶
包装规格: 100毫克/瓶 1瓶
计价单位:盒
生产厂家英文名:CELGENE CORPORATION
原产地英文商品名:VIDAZA 100MG SDV PWD 1/EA
原产地英文药品名:AZACITIDINE
中文参考商品译名:维达扎 100毫克/瓶 1瓶
中文参考药品译名:阿扎胞苷
曾用名:阿扎胞苷、5-氮杂胞苷、5-氮杂胞嘧啶核苷、氮胞苷、氮杂胞苷、Ladakamycin
简介:
部分中文维达扎处方资料(仅供参考)英文药名: Vidaza(Azacitidine Injection)中文药名: 维达扎(阿扎胞苷注射液)药品介绍阿扎胞苷(Azacitidine)英文名称:Azacitidine英文别名:5-Azacytidine、Ladakamycin其他名:5aza-C,拉达卡霉素(Ladakamycin),5-氮杂胞苷、5-氮杂胞嘧啶核苷、氮胞苷、氮杂胞苷。 作用机制:嘧啶类抗代谢药,干扰核苷酸的合成,以假嘧啶形式掺入DNA和RNA中,并与之结合。 适应症:急性非淋巴细胞性白血病。用于乳腺癌、肠癌、黑色素瘤等有一定疗效。 常用剂量:100mg/m2,静脉推注,每8小时一次,连用5天,200mg/(m2·d)静脉持续滴注,连用5天。 注意事项:由于药物的稳定性差,因此应该在用药前配药,配药后立即使用,配药后8小时未用则应丢弃。静脉滴注应该用新鲜林格液配制,每8小时配制一次。 毒性反应:(1)骨髓抑制和其他血液学反应:所有的病人都会出现严重的骨髓抑制和其他血液学反应,表现为白细胞于第12~14天降至最低,偶见抑制持续超过几周。(2)恶心呕吐和其他胃肠道反应:常见。静脉持续滴注可减轻恶心呕吐和其他胃肠道反应反应。(3)皮肤粘膜反应:粘膜炎及皮肤红疹偶见(4)其他反应:1)腹泻:常见。2)神经系统反应:肌肉疼痛、虚弱、嗜睡及昏迷,少见。3)肝毒性:罕见,但可能严重。4)暂时性发热:偶见。 FDA于04年5月19日宣布批准Vidaza(商品名:维达扎;通用名:azacitidine<阿扎胞苷>)注射液为治疗骨髓增生异常综合症(Myelodysplastic Syndrome, MDS)的第一个有效药物。DA代理专员Lester M. Crawford博士说:“通过恢复骨髓细胞的正常生长和分化,该新药将向有此种罕见的并且在一些病例中恶化为白血病的那些患者提供一个非常需要的治疗手段。FDA将继续给予这类具有显著疗效的产品的批准以最高的优先权。”MDS是由骨髓细胞功能异常、正常血细胞生成减少而引起的一系列疾病的总称。MDS可由治疗其它疾病的药物或放射治疗所致,也可由未知病因引起。MDS的某些类型会恶化为急性髓细胞样白血病(AML)。AML是白细胞增生过度活跃的一种癌症。FAB(法国-美国-英国)协作组织将MDS分为5型,分别是:顽固性贫血(RA)、环形铁粒幼红细胞性难治性贫血(RARS)、原始细胞过多性难治性贫血(RAEB)、转化型原始细胞过多性难治性贫血(RAEB-T)和慢性粒单核细胞白血病(CMMoL)。Vidaza属于罕见病治疗药。罕见病治疗药用于治疗患者人数在美国少于20万的罕见疾病或症状。《罕见病药物法》规定:获得所指定罕见病药物上市批准的首个申报者将享有该药物在美国市场上7年期的独占上市权。据估计,美国每年有7000-12,000的MDS新病例被确诊。这种疾病在各年龄段都可能发生,但是60岁以上人群发病率最高。典型症状包括虚弱、疲劳、感染、易淤伤、出血和发热。MDS患者可能需要接受红血球和血小板的输入,以及针对感染的抗生素治疗。英文版说明书:
Vidaza reviewVidaza is used to treat myelodysplastic syndrome (MDS), a type of bone marrow disease that typically precedes leukemia, and its subtypes such as various refractory anemia, including those with ringed sideroblasts, excess blasts, and excess blasts in transformation. MDS is caused by the improper functioning of the bone marrow. When bone marrow cells are deformed, this causes a decrease in blood production. It is also used in the treatment of some leukemia such as chronic myelomonocytic leukemia, refractory acute lymphocytic leukemia and refractory acute myelogenous leukemiaVidaza improves the bone marrow function by binding to the DNA and Ribonucleic acid, and improving blood production by killing off abnormal cells. In a study done on Vidaza use, patients that previously needed blood transfusions for MDS no longer needed it after being treated with Vidaza, and 16% reported full return of bone marrow structure and blood cell count.Vidaza is available as a powder, readily soluble in water and can be administered subcutaneously or intravenously by a physician or nurse. Treatment is typically a once-a-day injection for seven days, and may be repeated after 4 weeks. Physicians typically prescribe 4 treatments at a time. Doses may be increased after two cycles if significant improvement is not evident and no adverse side effects are seen. Alternatively, subsequent treatments may be delayed or dosages reduced if negative side effects are evident. It is important to communicate with your physician regarding any symptoms felt while undergoing treatment. The dosage is dependent on the patient's weight, previous treatment history and other assessments made by your physician. The physician may also use Vidaza in other related cases if found to work effectively.Known side effects may include nausea, diarrhea or constipation, mouth sores, tiredness, cramps, back and joint pain, perspiration, skin edema of ankles, hands and feet, rashes and reddening of skin and weakness. If you experience any of these symptoms, consult your physician.A low white blood count may be experienced in between sessions. During this period you may be susceptible to infections. This low point is temporary, lasting usually 10-17 days but possibly taking 28-31 days to recover.Serious side effects that require immediate medical attention include pale skin, rapid heartbeat, chest pains, bruising or bleeding, epistaxis, signs of infection such as a fever and sore throat, and red or purple dots on the skin. Consult your physician immediately if any of these symptoms occur.Make certain your physician is aware of any medications that you are currently taking as well as any medication allergies you have. Also, avoid taking aspirin while undergoing treatment unless your physician specifically allows this.Do not take any form of immunization such as vaccines while on Vidaza. The lowered white blood cell count may leave you in danger.Women who are pregnant or plan to become pregnant as well as women who are breastfeeding need to consult their doctor before taking this medication. Vidaza may be harmful to a developing fetus and may also affect fertility if you are planning to become pregnant. Avoid breastfeeding while on this medication.Contraceptives may also interact with the medication so use of barrier methods such as condoms are advised while taking this medication. Consult your physician for more information.Vidaza has the following structural formula:Molecular formula of vidaza is C8H12N4O5• Chemical IUPAC Name is 4-amino-1-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3,5-triazin-2-one• Molecular weight is 244.205 g/mol• Vidaza available : 100mg tablets.用药温馨提示:当您服用此药物时,需定期接受医疗专业人士的检查,以便随时针对其药效、副作用等情况进行监测。本网站所包含的信息旨在为患者提供帮助,不能代替医学建议和治疗。
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿扎胞苷注射剂AZACITIDINE(VIDAZA 100MG SDV PWD)
药品价格查询,专业药品查询网站,药品说明书查询,药品比价 » 阿扎胞苷注射剂AZACITIDINE(VIDAZA 100MG SDV PWD)